Abstract
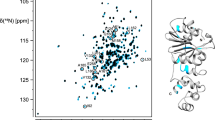
Sequence specific resonance assignments have been obtained for 1H, 13C and 15N nuclei of the 21 kDa (188 residues long) glutamine amido transferase subunit of guanosine monophosphate synthetase from Methanocaldococcus jannaschii. From an analysis of 1H and 13Cα, 13Cβ secondary chemical shifts, 3 JHNHα scalar coupling constants and sequential, short and medium range 1H–1H NOEs, it was deduced that the glutamine amido transferase subunit has eleven strands and five helices as the major secondary structural elements in its tertiary structure.


Similar content being viewed by others
References
Bodenhousen G, Ruben DJ (1980) Natural abundance nitrogen-15 NMR by enhanced heteronuclear spectroscopy. Chem Phys Lett 69:185–189
Cavanagh J, Fairbrother WJ, PalmerIII AG, Rance M, Skelton NJ (2007) Protein NMR spectroscopy: principles and practice. Academic Press, Inc, San Diego
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Grzesiek S, Bax A (1992a) Correlating backbone amide and side chain resonances in larger proteins by multiple relayed triple resonance NMR. J Am Chem Soc 114:6291–6293
Grzesiek S, Bax A (1992b) Improved 3d triple resonance techniques applied to 31 kDa protein. J Magn Reson 96:432–440
Grzesiek S, Bax A (1993) Amino acid type determination in the sequential assignment procedure of uniformly 13C/15N enriched proteins. J Biomol NMR 3:185–204
Huang X, Holden HM, Raushel FM (2001) Channeling of substrates and intermediates in enzyme-catalyzed reactions. Annu Rev Biochem 70:149–180
Lagerkvist U (1958) Biosynthesis of guanosine 5′-phosphate. II. Amination of xanthosine 5′-phosphate by purified enzyme from pigeon liver. J Biol Chem 233:143–149
Lewis EK, Ikura M, Tschudin R, Bax A (1990) Three-dimensional triple-resonance NMR spectroscopy of isotopically enriched proteins. J Magn Reson 89:496–514
Mori S, Abeygunawardana C, Johnson MO, van Zijl PC (1995) Improved sensitivity of HSQC spectra of exchanging protons at short interscan delays using a new fast HSQC (FHSQC) detection scheme that avoids water saturation. J Magn Reson B 108:94–98
Mouilleron S, Golinelli-Pimpaneau B (2007) Conformational changes in ammonia-channeling glutamine amidotransferases. Curr Opin Struct Biol 17:653–664
Muchmore DC, McIntosh LP, Russell CB, Anderson DE, Dahlquist FW (1989) Expression and nitrogen-15 labeling of proteins for proton and nitrogen-15 nuclear magnetic resonance. Methods Enzymol 177:44–73
Raushel FM, Thoden JB, Holden HM (2003) Enzymes with molecular tunnels. Acc Chem Res 36:539–548
van der Knaap JA, Kumar BR, Moshkin YM, Langenberg K, Krijgsveld J, Heck AJ, Karch F, Verrijzer CP (2005) GMP synthetase stimulates histone H2B deubiquitylation by the epigenetic silencer USP7. Mol Cell 17:695–707
van der Knaap JA, Kozhevnikova E, Langenberg K, Moshkin YM, Verrijzer CP (2010) Biosynthetic enzyme GMP synthetase cooperates with ubiquitin-specific protease 7 in transcriptional regulation of ecdysteroid target genes. Mol Cell Biol 30:736–744
von der Saal W, Crysler CS, Villafranca JJ (1985) Positional isotope exchange and kinetic experiments with Escherichia coli guanosine-5′-monophosphate synthetase. Biochemistry 24:5343–5350
Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59:687–696
Vuister GW, Bax A (1993) Quantitative J correlation: a new approach for measuring homonuclear three-bond J(HNHa) coupling constants in 15N-enriched proteins. J Am Chem Soc 115:7772–7777
Wishart D, Skyes B (1994) The 13C chemical shift index. A simple method for the identification of protein secondary structure using 13C chemical shift data. J Biomol NMR 4:171–180
Wittekind M, Muller L (1993) HNCACB, a high sensitive 3D NMR experiment to correlate amide-proton and nitrogen resonances with the alpha and beta carbon resonances in protein. J Magn Reson B 101:201–205
Zalkin H (1993) The amidotransferases. Adv Enzymol Relat Areas Mol Biol 66:203–309
Zalkin H, Murphy T (1975) Utilization of ammonia for tryptophan synthesis. Biochem Biophys Res Commun 67:1370–1377
Acknowledgments
SPS would like to thank DBT and DST for the NMR facilities at the Indian Institute of Science. RA would like to thank the Indian Institute of Science for a doctoral fellowship. HB would like to thank DBT and DST for funding. SK would like to thank CSIR for a research fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ali, R., Kumar, S., Balaram, H. et al. 1H, 13C, 15N assignment and secondary structure determination of glutamine amido transferase subunit of gaunosine monophosphate synthetase from Methanocaldococcus jannaschii . Biomol NMR Assign 6, 193–196 (2012). https://doi.org/10.1007/s12104-011-9354-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-011-9354-x