Abstract
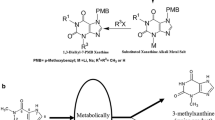
Paraxanthine (1,7-dimethylxanthine), a purine alkaloid derivative of caffeine (1,3,7-trimethylxanthine), is a high-value biochemical with several applications in the pharmaceutical and cosmetic industries. However, chemical synthesis of paraxanthine requires harsh conditions and frequently results in low yield mixtures of non-specifically N-methylated compounds. We have recently demonstrated that the mutant bacterial N-demethylase NdmA4 with its partner reductase NdmD is capable of producing paraxanthine as the major metabolite from caffeine. Here, we report the construction and screening of several Escherichia coli strains to produce paraxanthine from caffeine by means of whole-cell biocatalysts using varying dosages of ndmA4, ndmD, and the frmAB formaldehyde dehydrogenase genes. Preliminary resting cell assay results with the best paraxanthine-producing strain, MBM019, showed a 33% molar conversion of caffeine, from 5 mM to 3.35 mM, resulting in approximately 0.90 mM paraxanthine. However, a small amount of 7-methylxanthine was unexpectedly produced at a concentration of approximately 0.35 mM. After optimizing reaction conditions to a cellular concentration of OD600 = 50 and a caffeine concentration of 5 mM, the reaction was scaled-up to a volume of 620 mL, producing 1.02 mM paraxanthine and consuming 2.49 mM caffeine. The purified paraxanthine was then isolated via preparatory scale chromatography, resulting in 104.1 mg of product at high purity. This is the first reported strain genetically optimized for the biosynthetic production of paraxanthine.
Similar content being viewed by others
References
Franco, R., A. Oñatibia-Astibia, and E. Martínez-Pinilla (2013) Health benefits of methylxanthines in cacao and chocolate. Nutrients. 5: 4159–4173.
Singh, N., A. K. Shreshtha, M. S. Thakur, and S. Patra (2018) Xanthine scaffold: scope and potential in drug development. Heliyon. 4: e00829.
Constantin, S., F. G. Lupascu, M. Apotrosoaei, I. M. Vasincu, D. Lupascu, F. Buron, S. Routier, and L. Profire (2017) Synthesis and biological evaluation of the new 1,3-dimethylxanthlne derivatives with thiazolidine-4-one scaffold. Chem. Cent. J. 11: 12.
Xu, K., Y.-H. Xu, J.-F. Chen, and M. A. Schwarzschild (2010) Neuroprotection by caffeine: time course and role of its metabolites in the MPTP model of Parkinson’s disease. Neuroscience. 167: 475–481.
Janitschke, D., A. A. Lauer, C. M. Bachmann, H. S. Grimm, T. Hartmann, and M. O. Grimm (2021) Methylxanthines and neurodegenerative diseases: an update. Nutrients. 13: 803.
Oñatibia-Astibia, A., R. Franco, and E. Martínez-Pinilla (2017) Health benefits of methylxanthines in neurodegenerative diseases. Mol. Nutr. Food Res. 61: 1600670.
Negida, A., M. Elfil, A. Attia, E. Farahat, M. Gabr, A. Essam, D. Attia, and H. Ahmed (2017) Caffeine; the forgotten potential for Parkinson’s disease. CNS Neurol. Disord. Drug Targets. 16: 652–657.
Ross, G. W., R. D. Abbott, H. Petrovitch, D. M. Morens, A. Grandinetti, K.-H. Tung, C. M. Tanner, K. H. Masaki, P. L. Blanchette, J. D. Curb, J. S. Popper, and L. R. White (2000) Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA. 283: 2674–2679.
Ponte, B., M. Pruijm, D. Ackermann, G. Ehret, N. Ansermot, J. A. Staessen, B. Vogt, A. Pechère-Bertschi, M. Burnier, P.-Y. Martin, C. B. Eap, M. Bochud, and I. Guessous (2018) Associations of urinary caffeine and caffeine metabolites with arterial stiffness in a large population-based study. Mayo Clin. Proc. 93: 586–596.
Scurachio, R. S., F. Mattiucci, W. G. Santos, L. H. Skibsted, and D. R. Cardoso (2016) Caffeine metabolites not caffeine protect against riboflavin photosensitized oxidative damage related to skin and eye health. J. Photochem. Photobiol. B 163: 277–283.
Nunnari, G., E. Argyris, J. Fang, K. E. Mehlman, R. J. Pomerantz, and R. Daniel (2005) Inhibition of HIV-1 replication by caffeine and caffeine-related methylxanthines. Virology. 335: 177–184.
Lee, I.-A., A. Kamba, D. Low, and E. Mizoguchi (2014) Novel methylxanthine derivative-mediated anti-inflammatory effects in inflammatory bowel disease. World J. Gastroenterol. 20: 1127–1138.
Singh, H., N. S. Sahajpal, H. Singh, V. Vanita, P. Roy, S. Paul, S. K. Singh, I. Kaur, and S. K. Jain (2021) Pre-clinical and cellular toxicity evaluation of 7-methylxanthine: an investigational drug for the treatment of myopia. Drug Chem. Toxicol. 44: 575–584.
Ferré, S., M. Orrú, and X. Guitart (2013) Paraxanthine: connecting caffeine to nitric oxide neurotransmission. J. Caffeine Res. 3: 72–78.
Okuro, M., N. Fujiki, N. Kotorii, Y. Ishimaru, P. Sokoloff, and S. Nishino (2010) Effects of paraxanthine and caffeine on sleep, locomotor activity, and body temperature in orexin/ataxin-3 transgenic narcoleptic mice. Sleep. 33: 930–942.
Orrú, M., X. Guitart, M. Karcz-Kubicha, M. Solinas, Z. Justinova, S. K. Barodia, J. Zanoveli, A. Cortes, C. Lluis, V. Casado, F. G. Moeller, and S. Ferré (2013) Psychostimulant pharmacological profile of paraxanthine, the main metabolite of caffeine in humans. Neuropharmacology. 67: 476–484.
Negus, S. S. and L. L. Miller (2014) Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol. Rev. 66: 869–917.
Guerreiro, S., D. Toulorge, E. Hirsch, M. Marien, P. Sokoloff, and P. P. Michel (2008) Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol. Pharmacol. 74: 980–989.
Klemmer, I., S. Yagi, and O. A. Gressner (2011) Oral application of 1,7-dimethylxanthine (paraxanthine) attenuates the formation of experimental cholestatic liver fibrosis. Hepatol. Res. 41: 1094–1109.
Geraets, L., A. Haegens, A. R. Weseler, K. Brauers, J. H. Vernooy, E. F. Wouters, A. Bast, and G. J. Hageman (2010) Inhibition of acute pulmonary and systemic inflammation by 1,7-dimethylxanthine. Eur. J. Pharmacol. 629: 132–139.
Trier, K., S. Munk Ribel-Madsen, D. Cui, and S. Brøgger Christensen (2008) Systemic 7-methylxanthine in retarding axial eye growth and myopia progression: a 36-month pilot study. J. Ocul. Biol. Dis. Infor. 1: 85–93.
Bertrand, B., G. M. M. Groothuis, A. Casini, S. Loic, E. Bodio, R. Philippe, P. Legendre, D. Monchaud, and M. Picquet (2014) Xanthine-based gold(I) N-heterocyclic carbene complexes: synthesis and anticancer evaluation. J. Biol. Inorg. Chem. 19: S589.
Mohamed, H. A., B. R. Lake, T. Laing, R. M. Phillips, and C. E. Willans (2015) Synthesis and anticancer activity of silver(I)-N-heterocyclic carbene complexes derived from the natural xanthine products caffeine, theophylline and theobromine. Dalton Trans. 44: 7563–7569.
Szadkowska, A., S. Staszko, E. Zaorska, and R. Pawlowski (2016) A theophylline based copper N-heterocyclic carbene complex: synthesis and activity studies in green media. RSC Adv. 6: 44248–44253.
Valdés, H., D. Canseco-González, J. M. Germán-Acacio, and D. Morales-Morales (2018) Xanthine based N-heterocyclic carbene (NHC) complexes. J. Organomet. Chem. 867: 51–54.
Zhang, J.-J., C.-M. Che, and I. Ott (2015) Caffeine derived platinum(II) N-heterocyclic carbene complexes with multiple anti-cancer activities. J. Organomet. Chem. 782: 37–41.
Stavric, B. (1988) Methylxanthines: toxicity to humans. 3. Theobromine, paraxanthine and the combined effects of methylxanthines. Food Chem. Toxicol. 26: 725–733.
Müller, C. E., D. Deters, A. Dominik, and M. Pawlowski (1998) Synthesis of paraxanthine and isoparaxanthine analogs (1,7- and 1,9-substituted xanthine derivatives). Synthesis (Stuttg.) 1998: 1428–1436.
Gulevskaya, A. V. and A. F. Pozharskii (1991) Synthesis of N-substituted xanthines (review). Chem. Heterocycl. Compd. (N. Y.) 27: 1–23.
He, R., S. M. Ching, and Y. Lam (2006) Traceless solid-phase synthesis of substituted xanthines. J. Comb. Chem. 8: 923–928.
Zavialov, I. A., V. H. Dahanukar, H. Nguyen, C. Orr, F. Zhang, and D. R. Andrews (2004) New and practical method for synthesis of 1- and 1,3-substituted xanthines. Org. Lett. 6: 2237–2240. (Erratum published 2004, Org. Lett. 6: 3017)
Müller, C. E., D. Shi, M. Manning Jr., and J. W. Daly (1993) Synthesis of paraxanthine analogs (1,7-disubstituted xanthines) and other xanthines unsubstituted at the 3-position: structure-activity relationships at adenosine receptors. J. Med. Chem. 36: 3341–3349.
Ashengroph, M. (2017) Salinivibrio costicola GL6, a novel isolated strain for biotransformation of caffeine to theobromine under hypersaline conditions. Curr. Microbiol. 74: 34–41.
Summers, R. M., T. M. Louie, C. L. Yu, and M. Subramanian (2011) Characterization of a broad-specificity non-haem iron N-demethylase from Pseudomonas putida CBB5 capable of utilizing several purine alkaloids as sole carbon and nitrogen source. Microbiology (Reading). 157: 583–592.
Dash, S. S. and S. N. Gummadi (2010) Biodegradation of caffeine by Pseudomonas sp. NCIM 5235. Res. J. Microbiol. 5: 745–753.
Yu, C. L., T. M. Louie, R. Summers, Y. Kale, S. Gopishetty, and M. Subramanian (2009) Two distinct pathways for metabolism of theophylline and caffeine are coexpressed in Pseudomonas putida CBB5. J. Bacteriol. 191: 4624–4632.
Quandt, E. M., R. M. Summers, M. V. Subramanian, and J. E. Barrick (2015) Draft genome sequence of the bacterium Pseudomonas putida CBB5, which can utilize caffeine as a sole carbon and nitrogen source. Genome Announc. 3: e00640–15.
Summers, R. M., T. M. Louie, C.-L. Yu, L. Gakhar, K. C. Louie, and M. Subramanian (2012) Novel, highly specific N-demethylases enable bacteria to live on caffeine and related purine alkaloids. J. Bacteriol. 194: 2041–2049.
Summers, R., S. Gopishetty, S. Mohanty, and M. Subramanian (2014) New genetic insights to consider coffee waste as feedstock for fuel, feed, and chemicals. Cent. Eur. J. Chem. 12: 1271–1279.
Summers, R. M., J. L. Seffernick, E. M. Quandt, C. L. Yu, J. E. Barrick, and M. V. Subramanian (2013) Caffeine junkie: an unprecedented glutathione S-transferase-dependent oxygenase required for caffeine degradation by Pseudomonas putida CBB5. J. Bacteriol. 195: 3933–3939.
Algharrawi, K. H., R. M. Summers, S. Gopishetty, and M. Subramanian (2015) Direct conversion of theophylline to 3-methylxanthine by metabolically engineered E. coli. Microb. Cell Fact. 14: 203.
Algharrawi, K. H. R., R. M. Summers, and M. Subramanian (2017) Production of theobromine by N-demethylation of caffeine using metabolically engineered E. coli. Biocatal. Agric. Biotechnol. 11: 153–160.
Algharrawi, K. H. R. and M. Subramanian (2020) Production of 7-methylxanthine from theobromine by metabolically engineered E. coli. Iraqi J. Chem. Pet. Eng. 21: 19–27.
Mock, M. B., S. Zhang, B. Pniak, N. Belt, M. Witherspoon, and R. M. Summers (2021) Substrate promiscuity of the NdmCDE N7-demethylase enzyme complex. Biotechnol. Notes. 2: 18–25.
Kim, J. H., B. H. Kim, S. Brooks, S. Y. Kang, R. M. Summers, and H. K. Song (2019) Structural and mechanistic insights into caffeine degradation by the bacterial N-demethylase complex. J. Mol. Biol. 431: 3647–3661.
Mills, S. B., M. B. Mock, and R. M. Summers (2021) Rational protein engineering of bacterial N-demethylases to create biocatalysts for the production of methylxanthines. https://doi.org/10.1101/2021.12.17.472166
MacWilliams, M. P. and M.-K. Liao (2006) Luria Broth (LB) and Luria Agar (LA) Media and Their Uses Protocol. American Society for Microbiology, Washington, DC, USA.
Held, D., K. Yaeger, and R. Novy (2003) New coexpression vectors for expanded compatibilities in E. coli. Innovations. 18: 4–6.
Sektas, M. and W. Szybalski (2002) Novel single-copy pETcoco vector with dual controls for amplification and expression. Innovations. 14: 10–12.
Salis, H. M. (2011) The ribosome binding site calculator. Methods Enzymol. 498: 19–42.
Retnadhas, S. and S. N. Gummadi (2018) Identification and characterization of oxidoreductase component (NdmD) of methylxanthine oxygenase system in Pseudomonas sp. NCIM 5235. Appl. Microbiol. Biotechnol. 102: 7913–7926.
Zhang, Y., Z. Huang, C. Du, Y. Li, and Z. Cao (2009) Introduction of an NADH regeneration system into Klebsiella oxytoca leads to an enhanced oxidative and reductive metabolism of glycerol. Metab. Eng. 11: 101–106.
Berríos-Rivera, S. J., G. N. Bennett, and K.-Y. San (2002) The effect of increasing NADH availability on the redistribution of metabolic fluxes in Escherichia coli chemostat cultures. Metab. Eng. 4: 230–237.
Denby, K. J., J. Iwig, C. Bisson, J. Westwood, M. D. Rolfe, S. E. Sedelnikova, K. Higgins, M. J. Maroney, P. J. Baker, P. T. Chivers, and J. Green (2016) The mechanism of a formaldehyde-sensing transcriptional regulator. Sci. Rep. 6: 38879.
Chen, N. H., K. Y. Djoko, F. J. Veyrier, and A. G. McEwan (2016) Formaldehyde stress responses in bacterial pathogens. Front. Microbiol. 7: 257.
Gutheil, W. G., E. Kasimoglu, and P. C. Nicholson (1997) Induction of glutathione-dependent formaldehyde dehydrogenase activity in Escherichia coli and Hemophilus influenza. Biochem. Biophys. Res. Commun. 238: 693–696.
Herring, C. D. and F. R. Blattner (2004) Global transcriptional effects of a suppressor tRNA and the inactivation of the regulator frmR. J. Bacteriol. 186: 6714–6720.
Szolomájer, J., G. Paragi, G. Batta, C. F. Guerra, F. M. Bickelhaupt, Z. Kele, P. Pádár, Z. Kupihár, and L. Kovács (2011) 3-Substituted xanthines as promising candidates for quadruplex formation: computational, synthetic and analytical studies. New J. Chem. 35: 476–482.
Hergueta, A. R., M. J. Figueira, C. López, O. Caamaño, F. Fernández, and J. E. Rodríguez-Borges (2002) Synthesis of series of 1- and 3-differently substituted xanthines from imidazoles. Chem. Pharm. Bull. (Tokyo) 50: 1379–1382.
He, R. and Y. Lam (2005) A highly efficient solid-phase synthesis of 1,3-substituted xanthines. J. Comb. Chem. 7: 916–920.
Müller, C. E. and J. Sandoval-Ramírez (1995) A new versatile synthesis of xanthines with variable substituents in the 1-, 3-, 7-and 8-positions. Synthesis (Stuttg.) 1995: 1295–1299.
McKeague, M., Y.-H. Wang, A. Cravens, M. N. Win, and C. D. Smolke (2016) Engineering a microbial platform for de novo biosynthesis of diverse methylxanthines. Metab. Eng. 38: 191–203.
Acknowledgements
The authors thank Dr. Ken Belmore and the University of Alabama Department of Chemistry and Biochemistry for assistance with the NMR.
This work was supported by University of Alabama research funds. M.B. Mock is supported by the U.S. Department of Education as a GAANN Fellow (P200A180056).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no competing interests.
Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Mock, M.B., Mills, S.B., Cyrus, A. et al. Biocatalytic Production and Purification of the High-value Biochemical Paraxanthine. Biotechnol Bioproc E 27, 640–651 (2022). https://doi.org/10.1007/s12257-021-0301-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-021-0301-0