Abstract
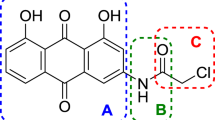
A series of 2-substituted-1,4-bis(dimethylamino)-9,10-anthraquinone derivatives were synthesized and their in vitro antiproliferative activities against p388 mouse leukemic tumor cells were evaluated. In addition, the effect of substituents on the phenyl ring was investigated. Among the derivatives tested, seven showed a high antiproliferative effect and three showed a moderate effect. In addition, introduction of a series of substituted phenyl groups into 1,4-bis(dimethylamino)-9,10-anthraquinone at 2-position were shown to enhance its antiproliferative activity. The antiproliferative activity also increased upon substitution of the benzene ring by an electron donating group such as an amine or methoxyl group.
Similar content being viewed by others
References
Berthiaume, J. M. and Wallace, K. B., Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol. Toxicol., 23, 15–25 (2007).
Capranico, G., Palumbo, M., Tinelli, S., Mabilia, M., Pozzan, A., and Zunino, F., Conformational drug determinants of the sequence specificity of drug-stimulated topoisomerase II DNA cleavage. J. Mol. Biol., 235, 1218–1230 (1994).
Cheng, C. C. and Zee-Cheng, R. K., The design, synthesis and development of a new class of potent antineoplastic anthraquinones. Prog. Med. Chem., 20, 83–118 (1983).
Dal Ben, D., Palumbo, M., Zagotto, G., Capranico, G., and Moro, S., DNA topoisomerase II structures and anthracycline activity: insights into ternary complex formation. Curr. Pharm. Des., 13, 2766–2780 (2007).
Fisher, G. R., Gutierrez, P. L., Oldcorne, M. A., and Patterson, L. H., NAD(P)H (quinone acceptor) oxidoreductase (DT-diaphorase)-mediated two-electron reduction of anthraquinone-based antitumour agents and generation of hydroxyl radicals. Biochem. Pharmacol., 43, 575–585 (1992).
Gatto, B., Zagotto, G., Sissi, C., Cera, C., Uriarte, E., Palù, G., Capranico, G., and Palumbo, M., Peptidyl anthraquinones as potential antineoplastic drugs: synthesis, DNA binding, redox cycling, and biological activity. J. Med. Chem., 39, 3114–3122 (1996).
Hsiao, C. J., Li, T. K., Chan, Y. L., Hsin, L. W., Liao, C. H., Lee, C. H., Lyu, P. C., and Guh, J. H., WRC-213, an l-methionine-conjugated mitoxantrone derivative, displays anticancer activity with reduced cardiotoxicity and drug resistance: identification of topoisomerase II inhibition and apoptotic machinery in prostate cancers. Biochem. Pharmacol., 75, 847–856 (2008).
Jin, G. Z., Kim, Y., Chung, J. H., Sok, D. E., and Ahn, B. Z., Anthracene-1,4,9,10-tetraone derivatives: synthesis and antitumor activity. Arch. Pharm. (Weinheim), 331, 380–384 (1998a).
Jin, G. Z., Song, G. Y., Zheng, X. G., Kim, Y., Sok, D. E., and Ahn, B. Z., 2-(1-Oxyalkyl)-1,4-dioxy-9,10-anthraquinones: synthesis and evaluation of antitumor activity. Arch. Pharm. Res., 21, 198–206 (1998b).
Jin, G. Z., You, Y. J., and Ahn, B. Z., Esters of 2-(1-hydroxyalkyl)-1,4-dihydroxy-9,10-anthraquinones with melphalan as multifunctional anticancer agents. Bioorg. Med. Chem. Lett., 11, 1473–1476 (2001a).
Jin, G. Z., You, Y. J., Kim, Y., Nam, N. H., and Ahn, B. Z., Esters of chlorambucil with 2-substituted 1,4-dihydroxy-9,10-anthraquinones as multifunctional anticancer agents. Eur. J. Med. Chem., 36, 361–366 (2001b).
Loadman, P. M., Swaine, D. J., Bibby, M. C., Welham, K. J., and Patterson, L. H., A preclinical pharmacokinetic study of the bioreductive drug AQ4N. Drug Metab. Dispos., 29, 422–426 (2001).
Perchellet, E. M., Magill, M. J., Huang, X., Dalke, D. M., Hua, D. H., and Perchellet, J. P., 1,4-Anthraquinone: an anticancer drug that blocks nucleoside transport, inhibits macromolecule synthesis, induces DNA fragmentation, and decreases the growth and viability of L1210 leukemic cells in the same nanomolar range as daunorubicin in vitro. Anticancer Drugs, 11, 339–352 (2000).
Piedade, J. A., Fernandes, I. R., and Oliveira-Brett, A. M., Electrochemical sensing of DNA-adriamycin interactions. Bioelectrochemistry, 56, 81–83 (2002).
Sadeghi-Aliabadi, H., Tabarzadi, M., and Zarghi, A., Synthesis and cytotoxic evaluation of two novel anthraquinone derivatives. Farmaco, 59, 645–649 (2004).
Silverman, R. B., The Organic Chemistry of Drug Design and Drug Actionf. Elsevier Academic Press, USA, (2004).
Tam, M. N., Nam, N. H., Jin, G. Z., Song, G. Y., and Ahn, B. Z., Synthesis and evaluation of the antitumor activity of 2-substituted 1,4-dihydroxy-9,10-anthraquinones. Arch. Pharm. (Weinheim), 333, 189–194 (2000).
Teich, L., Daub, K. S., Krügel, V., Nissler, L., Gebhardt, R., and Eger, K., Synthesis and biological evaluation of new derivatives of emodin. Bioorg. Med. Chem., 15, 5961–5971 (2004).
Trieb, M., Rauch, C., Wellenzohn, B., Wibowo, F., Loerting, T., Mayer, E., and Liedl, K. R., Daunomycin intercalation stabilizes distinct backbone conformations of DNA. J. Biomol. Struct. Dyn., 21, 713–724 (2004).
Tütem, E., Apak, R., and Sözgen, K., The interaction of antitumor-active anthraquinones with biologically important redox couples: I. Spectrophotometric investigation of the interaction of carminic acid and mitoxantrone with the iron (II, III) and copper (I, II) redox couples. J. Inorg. Biochem., 61, 79–96 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, GZ., Jin, HS. & Jin, LL. Synthesis and antiproliferative activity of 1,4-bis(dimethylamino)-9,10-anthraquinone derivatives against P388 mouse leukemic tumor cells. Arch. Pharm. Res. 34, 1071–1076 (2011). https://doi.org/10.1007/s12272-011-0704-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-011-0704-0