Abstract
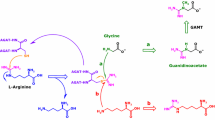
A rapid analytical method was developed to quantify dibasic amino acids (ornithine, lysine and arginine) after two-step derivatization procedure with good sensitivity and specificity on human plasma. If early diagnosis has not been made, patients with inborn metabolic disorders such as HHH syndrome, Hyperornithinemia and dibasic aminoaciduria rapidly progress to sudden death, physical defect or mental retardation resulting in storage of the toxic material into the brain. Therefore, it is necessary to develop the analytical method for rapid screening and/or correct confirmation diagnosis. The formation of trimethylsilyl derivative of the carboxylic (COO–) functional group was performed by adding MSTFA. Five μL of methyl orange was added to the residue until the color changed into yellow. Consecutively, the trifluoroacyl derivative of the amino (–NH2) functional group was produced by adding MBTFA. Specific ions was chosen for quantification with following ions; m/z 166 and m/z 212 for ornithine, m/z 180 and m/z 395 for lysine, and m/z 292 and, m/z 519 for arginine. A calibration curve showed a linear relationship for the dibasic amino acids spiked to pooled normal plasma showing R2 of 0.9955–0.9979 in the range of 0.1–600 ng investigated. The utility of the method for screening and diagnosis was demonstrated by recovery 80–125 % and reproducibility with RSD (9–17 %) at low, medium and high concentration fortified to pooled plasma. Collectively, the present study suggest that this method could be useful for diagnosis, screening, therapeutic monitoring of metabolic disorders on dietary therapy with excellent sensitivity and rapidity.






Similar content being viewed by others
References
Carvalho, D.R., J.M. Brum, C.E. Speck-Martins, F.D. Ventura, M.M. Navarro, K.E. Coelho, D. Portugal, and R. Pratesi. 2012. Clinical features and neurologic progression of hyperargininemia. Pediatric Neurology 46: 369–374.
Chillarón, J., M. Font-Llitjós, J. Fort, A. Zorzano, D.S. Goldfarb, V. Nunes, and M. Palacín. 2010. Pathophysiology and treatment of cystinuria. Nature Reviews Nephrology 6: 424–434.
Chiu, P.K., E.S. Chan, S.S. Hou, and C.F. Ng. 2008. Cystinuria: a rare diagnosis that should not be missed. Hong Kong Medical Journal 14: 399–401.
Jágr, M., J. Mráz, I. Linhart, V. Stránský, and M. Pospíšil. 2007. Synthesis and characterization of styrene oxide adducts with cysteine, histidine, and lysine in human globin. Chemical Research in Toxicology 20: 1442–1452.
Jeter, C.B., G.W. Hergenroeder, N.H. Ward 3rd, A.N. Moore, and P.K. Dash. 2012. Human traumatic brain injury alters circulating l-arginine and its metabolite levels: possible link to cerebral blood flow, extracellular matrix remodeling, and energy status. Journal of Neurotrauma 29: 119–127.
Jian, W.X., Q.H. Chen, and X.P. Huang. 2012. Metabolomics study of the myocardial tissue of rats of cardiac blood stasis syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi 32: 515–520.
Ko, J.M., C.H. Shin, S.W. Yang, M.W. Seong, S.S. Park, and J. Song. 2012. The first Korean case of lysinuric protein intolerance: presented with short stature and increased somnolence. Journal of Korean Medical Science 27: 961–964.
Korman, S.H., N. Kanazawa, B. Abu-Libdeh, A. Gutman, and S. Tsujino. 2004. Hyperornithinemia, hyperammonemia, and homocitrullinuria syndrome with evidence of mitochondrial dysfunction due to a novel SLC25A15 (ORNT1) gene mutation in a Palestinian family. Journal of the Neurological Sciences 218: 53–58.
Kuhara, T. 2002. Diagnosis and monitoring of inborn errors of metabolism using urease-pretreatment of urine, isotope dilution, and gas chromatography–mass spectrometry.). Journal of Chromatography B 781: 497–517.
Kuhara, T., M. Ohse, C. Ohdoi, and S. Ishida. 2000. Differential diagnosis of homocystinuria by urease treatment, isotope dilution and gas chromatography–mass spectrometry. Journal of Chromatography B 742: 59–70.
Kuhara, T., T. Shinka, Y. Inoue, M. Ohse, X. Zhen-wei, I. Yoshida, T. Inokuchi, S. Yamaguchi, M. Takayanagi, and I. Matsumoto. 1999. Pilot study of gas chromatographic-mass spectrometric screening of newborn urine for inborn errors of metabolism after treatment with urease. Journal of Chromatography B 731: 141–147.
Lu, Y., A. Ji-Ye, G. Wang, H. Hao, Q. Huang, B. Yan, M. Zhang, and K. Hao. 2008. Gas chromatography/time-of-flight mass spectrometry based metabolomic approach to differentiating hypertension- and age-related metabolic variation in spontaneously hypertensive rats. Rapid Communications in Mass Spectrometry 22(18): 2882–2888.
Ma, B., Q. Zhang, G.J. Wang, A. Ji-Ye, D. Wu, Y. Liu, B. Cao, L.S. Liu, Y.Y. Hu, Y.L. Wang, and Y.Y. Zheng. 2011. GC–TOF/MS-based metabolomic profiling of estrogen deficiency-induced obesity in ovariectomized rats. Acta Pharmacologica Sinica 32: 270–278.
Mao, Y.Y., J.Q. Bai, J.H. Chen, Z.F. Shou, Q. He, J.Y. Wu, Y. Chen, and Y.Y. Cheng. 2008. A pilot study of GC/MS-based serum metabolic profiling of acute rejection in renal transplantation. Transplant Immunology 19(1): 74–80.
Marco-Urrea, E., J. Seifert, M. von Bergen, and L. Adrian. 2012. Stable isotope peptide mass spectrometry to decipher amino acid metabolism in Dehalococcoides strain CBDB1. Journal of Bacteriology 194: 4169–4177.
Metges, C.C., and K.J. Petzke. 1997. Measurement of 15N/14N isotopic composition in individual plasma free amino acids of human adults at natural abundance by gas chromatography-combustion isotope ratio mass spectrometry. Analytical Biochemistry 247: 158–164.
Mühling, J., M.G. Dehne, M. Fuchs, A. Sablotzki, S. Weiss, J. Spatz, and G. Hempelmann. 2001. Conscientious metabolic monitoring on a patient with hyperornithinemia–hyperammonemia–homocitrullinuria (HHH) syndrome undergoing anaesthesia. Amino Acids 21: 303–318.
Noga, M.J., A. Dane, S. Shi, A. Attali, H. van Aken, E. Suidgeest, T. Tuinstra, B. Muilwijk, L. Coulier, T. Luider, T.H. Reijmers, R.J. Vreeken, and T. Hankemeier. 2012. Metabolomics of cerebrospinal fluid reveals changes in the central nervous system metabolism in a rat model of multiple sclerosis. Metabolomics 8(2): 253–263.
Ochi, H., H. Naito, K. Iwatsuki, T. Bamba, and E. Fukusaki. 2012. Metabolomics-based component profiling of hard and semi-hard natural cheeses with gas chromatography/time-of-flight-mass spectrometry, and its application to sensory predictive modeling. Journal of Bioscience and Bioengineering 113: 751–758.
Petrovic, R., J. Futas, J. Chandoga, and V. Jakus. 2005. Rapid and simple method for determination of Nepsilon-(carboxymethyl)lysine and Nepsilon-(carboxyethyl)lysine in urine using gas chromatography/mass spectrometry. Biomedical Chromatography 19: 649–654.
Redford-Ellis, M., A.C. Gibbs, S.A. Haider, and A. Holzel. 1981. Aminoaciduria in handicapped children: a study using ion-exchange chromatography as a screening test. Lancet 318: 10–15.
Saudubray, J.-M., and D. Rabier. 2007. Biomarkers identified in inborn errors for lysine, arginine, and ornithine. Journal of Nutrition 137: 1669S–1672S.
Sauer, S.W., S. Opp, G.F. Hoffmann, D.M. Koeller, J.G. Okun, and S. Kölker. 2011. Therapeutic modulation of cerebral l-lysine metabolism in a mouse model for glutaric aciduria type I. Brain 134: 157–170.
Schierbeek, H., K. Bijsterveld, T.E. Chapman, W.H. van Luijk, D.J. Reijngoud, and R. Berger. 1990. Stable isotope dilution analysis of cystine in granulocyte suspensions as cysteine: a powerful method for the diagnosis, the follow-up, and treatment of patients with cystinosis. Clinica Chimica Acta 191: 39–47.
Sebastio, G., M.P. Sperandeo, and G. Andria. 2011. Lysinuric protein intolerance: reviewing concepts on a multisystem disease. American Journal of Medical Genetics C 157(1): 54–62.
Serna, J.S.A., B.C. Sánchez, F.-F.M. Damas, and M.E. Valverde. 1993. An enteral modular formula in dibasic amino aciduria. Nutricion Hospitalaria 8: 441–446.
Shimizu, H., K. Maekawa, and Y. Eto. 1990. Abnormal urinary excretion of polyamines in HHH syndrome (hyperornithinemia associated with hyperammonemia and homocitrullinuria). Brain and Development 12: 533–535.
Smith, P.A., V. Villa, and G.L. King. 2010. Artifacts related to N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide derivatization of citrulline revealed by gas chromatography–mass spectrometry using both electron and chemical ionization. Journal of Chromatography A 1217(33): 5444–5448.
van Gelderen, H.H., and H.L. Teijema. 1973. Hyperlysinaemia. Harmless inborn error of metabolism? Archives of Disease in Childhood 48: 892–895.
Yoon, H.R. 2007. Two step derivatization for the analyses of organic, amino acids and glycines on filter paper plasma by GC–MS/SIM. Archives of Pharmacal Research 30(3): 387–395.
Acknowledgments
Author thanks to Hyunsu Jeong, Mirin Kang, and Heejoon Jeong for experiment and copy-editing a manuscript.
Conflict of interest
The author declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, HR. Determination of plasma dibasic amino acids following trimethylsilyl–trifluoroacyl derivatization using gas chromatography–mass spectrometry. Arch. Pharm. Res. 36, 366–373 (2013). https://doi.org/10.1007/s12272-013-0038-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0038-1