Abstract
Introduction
This in-vitro study compared the toxicity of bimatoprost 0.01% containing benzalkonium chloride (BAK) 0.02% with other commercial BAK-free or BAK-containing prostaglandin analogs.
Methods
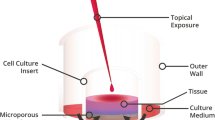
Six test solutions were evaluated: travoprost 0.004% with polyquaternium-1 0.001% (PQ), PQ, bimatoprost 0.01% with BAK 0.02%, latanoprost 0.005% with BAK 0.02%, tafluprost 0.0015% preservative free (PF), and BAK 0.02%. Phosphate-buffered saline (PBS) was the live control and 70% methanol was the dead control. Confluent human corneal epithelial cells were incubated with test solutions (diluted 1:5 or 1:10 with PBS) or control solutions for 10 or 25 min, after which cells were fluorescently labeled to distinguish live and dead cells. Data were expressed as a percentage of PBS live-cell fluorescence for automated readouts. Live and dead cells were manually counted for numeric analyses.
Results
For 1:5 and 1:10 dilutions using automated readout, cells exposed to bimatoprost with BAK, latanoprost with BAK, and BAK alone demonstrated significant reductions in the live cell signal compared with PBS, travoprost with PQ, and PQ alone (all P < 0.001). They also demonstrated significantly greater toxicity than tafluprost PF for 1:5 dilutions (all P < 0.001) and 1:10 dilutions (P ≤ 0.02), except for 1:10-diluted bimatoprost with BAK (P = 0.41). For 1:5 dilutions using manual cell count, cells exposed to bimatoprost with BAK demonstrated significant reductions in the percentage of live cells compared with PBS (P = 0.02). For 1:10 dilutions using manual cell count, cells exposed to bimatoprost with BAK, latanoprost with BAK, and BAK alone demonstrated significantly greater toxicity than PBS, travoprost with PQ, PQ alone, and tafluprost PF (all P ≤ 0.03). No significant differences were observed among PBS, travoprost with PQ, and PQ alone under any test conditions (P ≤ 0.63).
Conclusion
This study demonstrated that BAKcontaining solutions, including bimatoprost 0.01% with BAK, were toxic to human corneal epithelial cells, whereas BAK-free solutions showed little to no evidence of toxicity.
Similar content being viewed by others
References
American Academy of Ophthalmology. Primary open-angle glaucoma suspect, preferred practice pattern. San Francisco, 2005. Available at: http://www.aao.org/ppp. Accessed Jul 13 2012.
European Glaucoma Society. Terminology and guidelines for glaucoma. 3rd edition. Savona, Italy: Dogma; 2008.
Ammar DA, Noecker RJ, Kahook MY. Effects of benzalkonium chloride-preserved, polyquadpreserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv Ther. 2010;27:837–845.
Baudouin C, Riancho L, Warnet JM, Brignole F. In vitro studies of antiglaucomatous prostaglandin analogues: travoprost with and without benzalkonium chloride and preserved latanoprost. Invest Ophthalmol Vis Sci. 2007;48:4123–4128.
Brignole-Baudouin F, Riancho L, Liang H, Baudouin C. Comparative in vitro toxicology study of travoprost polyquad-preserved, travoprost BAK-preserved, and latanoprost BAK-preserved ophthalmic solutions on human conjunctival epithelial cells. Curr Eye Res. 2011;36:979–988.
Guenoun JM, Baudouin C, Rat P, Pauly A, Warnet JM, Brignole-Baudouin F. In vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatoprost in conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:2444–2450.
Liang H, Baudouin C, Pauly A, Brignole-Baudouin F. Conjunctival and corneal reactions in rabbits following short- and repeated exposure to preservative-free tafluprost, commercially available latanoprost and 0.02% benzalkonium chloride. Br J Ophthalmol. 2008;92:1275–1282.
Liang H, Baudouin C, Faure MO, Lambert G, Brignole-Baudouin F. Comparison of the ocular tolerability of a latanoprost cationic emulsion versus conventional formulations of prostaglandins: an in vivo toxicity assay. Mol Vis. 2009;15:1690–1699.
Liang H, Pauly A, Riancho L, Baudouin C, Brignole-Baudouin F. Toxicological evaluation of preservative-containing and preservativefree topical prostaglandin analogues on a threedimensional-reconstituted corneal epithelium system. Br J Ophthalmol. 2011;95:869–875.
Pellinen P, Huhtala A, Tolonen A, Lokkila J, Mäenpää J, Uusitalo H. The cytotoxic effects of preserved and preservative-free prostaglandin analogs on human corneal and conjunctival epithelium in vitro and the distribution of benzalkonium chloride homologs in ocular surface tissues in vivo. Curr Eye Res. 2012;37:145–154
Kahook MY, Noecker R. Quantitative analysis of conjunctival goblet cells after chronic application of topical drops. Adv Ther. 2008;25:743–751.
Kahook MY, Noecker RJ. Comparison of corneal and conjunctival changes after dosing of travoprost preserved with sofZia, latanoprost with 0.02% benzalkonium chloride, and preservativefree artificial tears. Cornea. 2008;27:339–343.
Whitson JT, Cavanagh HD, Lakshman N, Petroll WM. Assessment of corneal epithelial integrity after acute exposure to ocular hypotensive agents preserved with and without benzalkonium chloride. Adv Ther. 2006;23:663–671.
Cvenkel B, Ihan A. Ocular surface changes induced by topical antiglaucoma monotherapy. Ophthalmologica. 2002;216:175–179.
Guglielminetti E, Barabino S, Monaco M, Mantero S, Rolando M. HLA-DR expression in conjunctival cells after latanoprost. J Ocul Pharmacol Ther. 2002;18:1–9.
Martone G, Frezzotti P, Tosi GM, et al. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am J Ophthalmol. 2009;147:725–735.
Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–334.
Lumigan (bitmatoproset ophthalmic solution) [package insert]. Irvine, CA: Allergan, Inc.; 2010.
Majumdar S, Hippalgaonkar K, Repka MA. Effect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir across isolated rabbit cornea. Int J Pharm. 2008;348:175–178.
Robertson DM, Li L, Fisher S, et al. Characterization of growth and differentiation in a telomeraseimmortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46:470–478.
Yee RW, Norcom EG, Zhao XC. Comparison of the relative toxicity of travoprost 0.004% without benzalkonium chloride and latanoprost 0.005% in an immortalized human cornea epithelial cell culture system. Adv Ther. 2006;23:511–519.
Gandolfi S, Paredes T, Goldberg I, et al; Travoprost BAK-Free Clinical Study Group. Comparison of a travoprost BAK-free formulation preserved with polyquaternium-1 with BAK-preserved travoprost in ocular hypertension or open-angle glaucoma. Eur J Ophthalmol. 2012;22:34–44.
Kitazawa Y, Smith P, Sasaki N, Kotake S, Bae K, Iwamoto Y. Travoprost 0.004%/timolol 0.5%-fixed combination with and without benzalkonium chloride: a prospective, randomized, doubledmasked comparison of safety and efficacy. Eye (Lond). 2011;25:1161–1169.
Whitson JT, Trattler WB, Matossian C, Williams J, Hollander DA. Ocular surface tolerability of prostaglandin analogs in patients with glaucoma or ocular hypertension. J Ocul Pharmacol Ther. 2010;26:287–292.
Lewis RA, Katz GJ, Weiss MJ, et al. Travoprost BACFree Study Group. Travoprost 0.004% with and without benzalkonium chloride: a comparison of safety and efficacy. J Glaucoma. 2007;16:98–103.
Katz G, Springs CL, Craven ER, Montecchi-Palmer M. Ocular surface disease in patients with glaucoma or ocular hypertension treated with either BAK-preserved latanoprost or BAK-free travoprost. Clin Ophthalmol. 2010;4:1253–1261.
Aihara M, Otani S, Kozaki J, et al. Long-term effect of BAK-free travoprost on ocular surface and intraocular pressure in glaucoma patients after transition from latanoprost. J Glaucoma. 2012;21:60–64.
Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–355.
Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153:1–9.
Turacli E, Budak K, Kaur A, Mizrak B, Ekinci C. The effects of long-term topical glaucoma medication on conjunctival impression cytology. Int Ophthalmol. 1997;21:27–33.
Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29:618–621.
Author information
Authors and Affiliations
Corresponding author
Additional information
To view enhanced content go to www.advancesintherapy.com
Rights and permissions
About this article
Cite this article
Whitson, J.T., Petroll, W.M. Corneal Epithelial Cell Viability Following Exposure to Ophthalmic Solutions Containing Preservatives and/or Antihypertensive Agents. Adv Therapy 29, 874–888 (2012). https://doi.org/10.1007/s12325-012-0057-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-012-0057-1