Abstract
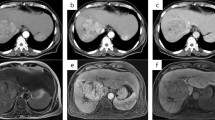
Atezolizumab plus bevacizumab (Atezo + Bev) is the first immunotherapy for hepatocellular carcinoma (HCC), and in the current guidelines, it is positioned as the first-line chemotherapy for unresectable cases. Herein, we report a case of HCC with pseudoprogression followed by a complete response to Atezo + Bev. A 56 year-old man was diagnosed with intermediate-stage HCC, as defined by the Barcelona Clinic Liver Cancer system stage B. Computed tomography (CT) revealed multiple lesions in the liver without any extrahepatic lesions. First, he was treated with transcatheter arterial chemoembolization (TACE); however, multiple residual lesions were observed on CT scan 2 months after TACE. Therefore, treatment with Atezo + Bev was initiated 4 months after TACE. After the third administration of Atezo + Bev, a CT scan showed progressive disease in intrahepatic lesions, along with increased serum levels of tumor markers. Although TACE was planned again, Atezo + Bev was continued while the patient was waiting for hospitalization. After the fifth administration of Atezo + Bev, serum levels of tumor markers decreased to the normal range. Magnetic resonance imaging showed prominently reduced tumor size. Therefore, Atezo + Bev was continued, and after the eighth administration, the CT scan showed the disappearance of all the liver lesions, indicating a complete response. In immunotherapy, the therapeutic response can sometimes be obtained in an atypical pattern due to either an increase in tumor burden or the appearance of new lesions, called “pseudoprogression,” which is rare in HCC.


Similar content being viewed by others
References
Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151–72.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–905.
Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862.
Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus durvalumab in unresectable hepatocellular Carcinoma. NEJM Evidence. 2022;1:EVIDo2100070.
Kelley RK, Rimassa L, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:995–1008.
Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol. 2020;6:e204564.
Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960–70.
Park HJ, Kim KW, Pyo J, et al. Incidence of pseudoprogression during immune checkpoint inhibitor therapy for solid tumors: a systematic review and meta-analysis. Radiology. 2020;297:87–96.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73.
Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66.
Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular Carcinoma. N Engl J Med. 2018;379:54–63.
Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–96.
Ippolito D, Maino C, Ragusi M, et al. Immune response evaluation criteria in solid tumors for assessment of atypical responses after immunotherapy. World J Clin Oncol. 2021;12:323–34.
Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33:3541–3.
Jia W, Gao Q, Han A, et al. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med. 2019;16:655–70.
Long GV, Weber JS, Larkin J, et al. Nivolumab for patients with advanced melanoma treated beyond progression: analysis of 2 phase 3 clinical trials. JAMA Oncol. 2017;3:1511–9.
Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84.
Katz SI, Hammer M, Bagley SJ, et al. Radiologic pseudoprogression during anti-PD-1 therapy for advanced non-small cell lung cancer. J Thorac Oncol. 2018;13:978–86.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35.
Escudier B, Motzer RJ, Sharma P, et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in checkmate 025. Eur Urol. 2017;72:368–76.
George S, Motzer RJ, Hammers HJ, et al. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a subgroup analysis of a randomized clinical trial. JAMA Oncol. 2016;2:1179–86.
Borcoman E, Kanjanapan Y, Champiat S, et al. Novel patterns of response under immunotherapy. Ann Oncol. 2019;30:385–96.
Mamdani H, Wu H, O’Neil BH, et al. Excellent response to Anti-PD-1 therapy in a patient with hepatocellular carcinoma: case report and review of literature. Discov Med. 2017;23:331–6.
Grierson P, Crites D, Ruzinova MB, et al. Distinct clinical and magnetic resonance features of metastatic hepatocellular carcinoma treated with pembrolizumab: a case report of late response after pseudoprogression. Hepatol Commun. 2018;2:148–51.
Watanabe Y, Ogawa M, Tamura Y, et al. A case of pseudoprogression in hepatocellular carcinoma treated with atezolizumab plus bevacizumab. J Investig Med High Impact Case Rep. 2021;9:23247096211058490.
Shigefuku R, Yoshikawa K, Tsukimoto M, et al. A Case of hepatocellular carcinoma pseudoprogression involving the main portal vein, right ventricular invasion, and exacerbation of lung metastases in a patient on atezolizumab plus bevacizumab. Intern Med. 2022. https://doi.org/10.2169/internalmedicine.9701-22.
Hales RK, Banchereau J, Ribas A, et al. Assessing oncologic benefit in clinical trials of immunotherapy agents. Ann Oncol. 2010;21:1944–51.
Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20.
Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19:3936–43.
Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52.
Hodi FS, Ballinger M, Lyons B, et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol. 2018;36:850–8.
Kataoka Y, Hirano K. Which criteria should we use to evaluate the efficacy of immune-checkpoint inhibitors? Ann Transl Med. 2018;6:222.
Lee JH, Long GV, Menzies AM, et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti-programmed cell death 1 antibodies. JAMA Oncol. 2018;4:717–21.
Sanmamed MF, Perez-Gracia JL, Schalper KA, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol. 2017;28:1988–95.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights
All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from the patient to be included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Odagiri, N., Tamori, A., Kotani, K. et al. A case of hepatocellular carcinoma with “pseudoprogression” followed by complete response to atezolizumab plus bevacizumab. Clin J Gastroenterol 16, 392–396 (2023). https://doi.org/10.1007/s12328-023-01761-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-023-01761-6