Abstract
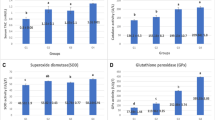
The present study was focused on evaluating the effects of Bacillus methylotrophicus SY200 in broiler production. A total of 120 healthy 7-day-old broiler chicks were randomly assigned to four dietary treatments, which included basal diet supplemented with 0%, 0.10%, 0.25%, or 0.50% (w/w) B. methylotrophicus SY200 preparation (1.0 × 109 cfu/g), regarded as negative control group (NC), low-dose group (BML), medium-dose group (BMM), and high-dose group (BMH), respectively. Each treatment was fed the corresponding experimental diet for 35 days. Results showed that dietary supplementation of B. methylotrophicus SY200 could improve broiler weight gain, especially the finisher phase. Further studies suggested that a certain amount of B. methylotrophicus SY200 enhanced the broiler antioxidant status and improved the morphological development of jejunum. Besides, dietary supplementation of B. methylotrophicus SY200 especially in 0.50% levels significantly increased the relative weight of immune organs and Newcastle disease virus antibody titer, similarly, increased mRNA expression levels of claudin-1, claudin-3, zonula occluden-1, and zonula occluden-2 were observed in the jejunum of BMM group. Moreover, B. methylotrophicus SY200 also showed beneficial effects in improving broilers microbiota homeostasis by increasing the number of beneficial bacteria. Conclusively, B. methylotrophicus SY200 could effectively improve the antioxidant status, modulate the intestinal structure, enhance the intestinal mucosal barrier function, and regulate the immune function of broilers, which finally improves the performance of the chicken in the finisher period.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Starr MP, Reynolds DM (1951) Streptomycin resistance of coliform bacteria from turkeys fed streptomycin. Am J Public Health Nations Health 41(11 Pt 1):1375–1380. https://doi.org/10.2105/ajph.41.11_pt_1.1375
Elliott SD, Barnes EM (1959) Changes in serological type and antibiotic resistance of Lancefield group D streptococci in chickens receiving dietary chlortetracycline. J Gen Microbiol 20(2):426–433. https://doi.org/10.1099/00221287-20-2-426
White AC Jr, Kang G (2015) Antibiotics, microbiota and health: are there dangers hiding in plain sight? Curr Opin Infect Dis 28(5):455–456. https://doi.org/10.1097/qco.0000000000000195
Song C, Li L, Zhang C, Qiu L, Fan L, Wu W, Meng S, Hu G, Chen J, Liu Y, Mao A (2017) Dietary risk ranking for residual antibiotics in cultured aquatic products around Tai Lake, China. Ecotoxicol Environ Saf 144:252–257. https://doi.org/10.1016/j.ecoenv.2017.06.036
Zhang Y, Hu Y, Deng S, Yuan Z, Li C, Lu Y, He Q, Zhou M, Deng R (2020) Engineering multivalence aptamer probes for amplified and label-free detection of antibiotics in aquatic products. J Agric Food Chem 68(8):2554–2561. https://doi.org/10.1021/acs.jafc.0c00141
Caglayan MO (2020) Aptamer-based ellipsometric sensor for ultrasensitive determination of aminoglycoside group antibiotics from dairy products. J Sci Food Agric 100(8):3386–3393. https://doi.org/10.1002/jsfa.10372
Salyers AA, Gupta A, Wang Y (2004) Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends Microbiol 12(9):412–416. https://doi.org/10.1016/j.tim.2004.07.004
Dyson ZA, Klemm EJ, Palmer S, Dougan G (2019) Antibiotic resistance and typhoid. Clin Infect Dis 68(Suppl 2):S165-170. https://doi.org/10.1093/cid/ciy1111
Yeom JR, Yoon SU, Kim CG (2017) Quantification of residual antibiotics in cow manure being spread over agricultural land and assessment of their behavioral effects on antibiotic resistant bacteria. Chemosphere 182:771–780. https://doi.org/10.1016/j.chemosphere.2017.05.084
Landman WJ, van Eck JH (2015) The incidence and economic impact of the Escherichia coli peritonitis syndrome in Dutch poultry farming. Avian Pathol 44(5):370–378. https://doi.org/10.1080/03079457.2015.1060584
Knap I, Lund B, Kehlet AB, Hofacre C, Mathis G (2010) Bacillus licheniformis prevents necrotic enteritis in broiler chickens. Avian Dis 54(2):931–935. https://doi.org/10.1637/9106-101509-ResNote.1
Lin Y, Xu S, Zeng D, Ni X, Zhou M, Zeng Y, Wang H, Zhou Y, Zhu H, Pan K, Li G (2017) Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. PLoS One 12(8):e0182426. https://doi.org/10.1371/journal.pone.0182426
Xu S, Lin Y, Zeng D, Zhou M, Zeng Y, Wang H, Zhou Y, Zhu H, Pan K, Jing B, Ni X (2018) Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci Rep 8(1):1744. https://doi.org/10.1038/s41598-018-20059-z
Lee H, Kim H-Y (2011) Lantibiotics, class I bacteriocins from the genus Bacillus. J Microbiol Biotechnol 21(3):229–235. https://doi.org/10.4014/jmb.1010.10017
Amoah K, Huang QC, Tan BP, Zhang S, Chi SY, Yang QH, Liu HY, Dong XH (2019) Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 87:796–808. https://doi.org/10.1016/j.fsi.2019.02.029
Madhaiyan M, Poonguzhali S, Kwon SW, Sa TM (2010) Bacillus methylotrophicus sp. nov., a methanol-utilizing, plant-growth-promoting bacterium isolated from rice rhizosphere soil. Int J Syst Evol Microbiol 60(Pt 10):2490–2495. https://doi.org/10.1099/ijs.0.015487-0
Radhakrishnan R, Lee IJ (2016) Gibberellins producing Bacillus methylotrophicus KE2 supports plant growth and enhances nutritional metabolites and food values of lettuce. Plant Physiol Biochem 109:181–189. https://doi.org/10.1016/j.plaphy.2016.09.018
Perez-Flores P, Valencia-Cantero E, Altamirano-Hernandez J, Pelagio-Flores R, Lopez-Bucio J, Garcia-Juarez P, Macias-Rodriguez L (2017) Bacillus methylotrophicus M4–96 isolated from maize (Zea mays) rhizoplane increases growth and auxin content in Arabidopsis thaliana via emission of volatiles. Protoplasma 254(6):2201–2213. https://doi.org/10.1007/s00709-017-1109-9
Cheng X, Ji X, Ge Y, Li J, Qi W, Qiao K (2019) Characterization of antagonistic Bacillus methylotrophicus isolated from rhizosphere and its biocontrol effects on maize stalk rot. Phytopathology 109(4):571–581. https://doi.org/10.1094/phyto-07-18-0220-r
Ji X, Li J, Meng Z, Zhang S, Dong B, Qiao K (2019) Synergistic effect of combined application of a new fungicide fluopimomide with a biocontrol agent Bacillus methylotrophicus TA-1 for management of gray mold in tomato. Plant Dis 103(8):1991–1997. https://doi.org/10.1094/pdis-01-19-0143-re
Frikha-Gargouri O, Ben Abdallah D, Ghorbel I, Charfeddine I, Jlaiel L, Triki MA, Tounsi S (2017) Lipopeptides from a novel Bacillus methylotrophicus 39b strain suppress Agrobacterium crown gall tumours on tomato plants. Pest Manag Sci 73(3):568–574. https://doi.org/10.1002/ps.4331
Jemil N, Ben Ayed H, Hmidet N, Nasri M (2016) Characterization and properties of biosurfactants produced by a newly isolated strain Bacillus methylotrophicus DCS1 and their applications in enhancing solubility of hydrocarbon. World J Microbiol Biotechnol 32(11):175. https://doi.org/10.1007/s11274-016-2132-2
Chaprao MJ, da Silva R, Rufino RD, Luna JM, Santos VA, Sarubbo LA (2018) Production of a biosurfactant from Bacillus methylotrophicus UCP1616 for use in the bioremediation of oil-contaminated environments. Ecotoxicology 27(10):1310–1322. https://doi.org/10.1007/s10646-018-1982-9
Sim I, Koh JH, Kim DJ, Gu SH, Park A, Lim YH (2015) In vitro assessment of the gastrointestinal tolerance and immunomodulatory function of Bacillus methylotrophicus isolated from a traditional Korean fermented soybean food. J Appl Microbiol 118(3):718–726. https://doi.org/10.1111/jam.12719
Upadhaya SD, Shanmugam SK, Kang DK, Kim IH (2017) Preliminary assessment on potentials of probiotic B. subtilis RX7 and B. methylotrophicus C14 strains as an immune modulator in Salmonella-challenged weaned pigs. Trop Anim Health Prod 49(5):1065–1070. https://doi.org/10.1007/s11250-017-1278-8
Xiao D, Yang G, Wang Z, Khalique A, Zhu Z, Xiong L, Li J, Yuan X, Ni X, Zeng D, Zhang D, Pan K (2020) Efficacy of Bacillus methylotrophicus SY200 strain as feed additive against experimental Salmonella typhimurium infection in mice. Microb Pathog 141:103978. https://doi.org/10.1016/j.micpath.2020.103978
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Bartosch S, Woodmansey EJ, Paterson JCM, McMurdo MET, Macfarlane GT (2005) Microbiological effects of consuming a synbiotic containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose in elderly persons, determined by real-time polymerase chain reaction and counting of viable bacteria. Clin Infect Dis 40(1):28–37. https://doi.org/10.1086/426027
Wu Y, Zhen W, Geng Y, Wang Z, Guo Y (2019) Pretreatment with probiotic Enterococcus faecium NCIMB 11181 ameliorates necrotic enteritis-induced intestinal barrier injury in broiler chickens. Sci Rep 9(1):10256. https://doi.org/10.1038/s41598-019-46578-x
Osho SO, Adeola O (2019) Impact of dietary chitosan oligosaccharide and its effects on coccidia challenge in broiler chickens. Br Poult Sci 60(6):766–776. https://doi.org/10.1080/00071668.2019.1662887
Emami NK, Calik A, White MB, Young M, Dalloul RA (2019) Necrotic enteritis in broiler chickens: the role of tight junctions and mucosal immune responses in alleviating the effect of the disease. Microorganisms 7(8):231. https://doi.org/10.3390/microorganisms7080231
Denman SE, McSweeney CS (2006) Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol 58(3):572–582. https://doi.org/10.1111/j.1574-6941.2006.00190.x
Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K (2008) Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol 47(5):367–373. https://doi.org/10.1111/j.1472-765X.2008.02408.x
Bartosch S, Fite A, Macfarlane GT, McMurdo ME (2004) Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol 70(6):3575–3581. https://doi.org/10.1128/aem.70.6.3575-3581.2004
Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A (2004) Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol 97(6):1166–1177. https://doi.org/10.1111/j.1365-2672.2004.02409.x
Walter J, Hertel C, Tannock GW, Lis CM, Munro K, Hammes WP (2001) Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl Environ Microbiol 67(6):2578–2585. https://doi.org/10.1128/aem.67.6.2578-2585.2001
Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R (2004) Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol 70(12):7220–7228. https://doi.org/10.1128/aem.70.12.7220-7228.2004
Matsuki T, Watanabe K, Fujimoto J, Miyamoto Y, Takada T, Matsumoto K, Oyaizu H, Tanaka R (2002) Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl Environ Microbiol 68(11):5445–5451. https://doi.org/10.1128/aem.68.11.5445-5451.2002
Gong L, Wang B, Mei X, Xu H, Qin Y, Li W, Zhou Y (2018) Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim Sci J 89(11):1561–1571. https://doi.org/10.1111/asj.13089
Wang J, Ni X, Wen B, Zhou Y, Liu L, Zeng Y, Zhao W, Khalique A, Wang P, Pan K, Yu Z, Jing B, Liu H, Zeng D (2020) Bacillus strains improve growth performance via enhancing digestive function and anti-disease ability in young and weaning rex rabbits. Appl Microbiol Biotechnol 104(10):4493–4504. https://doi.org/10.1007/s00253-020-10536-9
Chen Y-C, Yu Y-H (2020) Bacillus licheniformis–fermented products improve growth performance and the fecal microbiota community in broilers. Poult Sci 99(3):1432–1443. https://doi.org/10.1016/j.psj.2019.10.061
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24(5):981–990. https://doi.org/10.1016/j.cellsig.2012.01.008
Celi P (2010) The role of oxidative stress in small ruminants’ health and production. Rev Bras Zootec 39:348–363. https://doi.org/10.1590/S1516-35982010001300038
Hirata Y (2019) Reactive oxygen species (ROS) signaling: regulatory mechanisms and pathophysiological roles. Yakugaku Zasshi 139(10):1235–1241. https://doi.org/10.1248/yakushi.19-00141
Lu P, Xue WY, Zhang XL, Wu DW, Ding LR, Wen C, Zhou YM (2019) Heat-induced protein oxidation of soybean meal impairs growth performance and antioxidant status of broilers. Poult Sci 98(1):276–286. https://doi.org/10.3382/ps/pey344
Miao Q, Si X, Xie Y, Chen L, Liu Z, Liu L, Tang X, Zhang H (2020) Effects of acute heat stress at different ambient temperature on hepatic redox status in broilers. Poult Sci 99(9):4113–4122. https://doi.org/10.1016/j.psj.2020.05.019
Zhang L, Bai K, Zhang J, Xu W, Huang Q, Wang T (2017) Dietary effects of Bacillus subtilis fmbj on the antioxidant capacity of broilers at an early age. Poult Sci 96(10):3564–3573. https://doi.org/10.3382/ps/pex172
Deng W, Dong XF, Tong JM, Zhang Q (2012) The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult Sci 91(3):575–582. https://doi.org/10.3382/ps.2010-01293
Chaudhari AA, Lee Y, Lillehoj HS (2020) Beneficial effects of dietary supplementation of Bacillus strains on growth performance and gut health in chickens with mixed coccidiosis infection. Vet Parasitol 277:109009. https://doi.org/10.1016/j.vetpar.2019.109009
Bai K, Huang Q, Zhang J, He J, Zhang L, Wang T (2017) Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult Sci 96(1):74–82. https://doi.org/10.3382/ps/pew246
Potten CS (1998) Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci 353(1370):821–830. https://doi.org/10.1098/rstb.1998.0246
Fan YK, Croom J, Christensen VL, Black BL, Bird AR, Daniel LR, McBride BW, Eisen EJ (1997) Jejunal glucose uptake and oxygen consumption in turkey poults selected for rapid growth. Poult Sci 76(12):1738–1745. https://doi.org/10.1093/ps/76.12.1738
Samanya M, Yamauchi K-e (2002) Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var. natto. Comp Biochem Physiol Part A Mol Integr Physiol 133(1):95–104. https://doi.org/10.1016/S1095-6433(02)00121-6
Xu ZR, Hu CH, Xia MS, Zhan XA, Wang MQ (2003) Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult Sci 82(6):1030–1036. https://doi.org/10.1093/ps/82.6.1030
Rajput IR, Li LY, Xin X, Wu BB, Juan ZL, Cui ZW, Yu DY, Li WF (2013) Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult Sci 92(4):956–965. https://doi.org/10.3382/ps.2012-02845
Sen S, Ingale SL, Kim YW, Kim JS, Kim KH, Lohakare JD, Kim EK, Kim HS, Ryu MH, Kwon IK, Chae BJ (2012) Effect of supplementation of Bacillus subtilis LS 1–2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res Vet Sci 93(1):264–268. https://doi.org/10.1016/j.rvsc.2011.05.021
Dong Y, Li R, Liu Y, Ma L, Zha J, Qiao X, Chai T, Wu B (2020) Benefit of dietary supplementation with Bacillus subtilis BYS2 on growth performance, immune response, and disease resistance of broilers. Probiotics Antimicrob Proteins 12(4):1385–1397. https://doi.org/10.1007/s12602-020-09643-w
Monson MS, Settlage RE, Mendoza KM, Rawal S, El-Nezami HS, Coulombe RA, Reed KM (2015) Modulation of the spleen transcriptome in domestic turkey (Meleagris gallopavo) in response to aflatoxin B1 and probiotics. Immunogenetics 67(3):163–178. https://doi.org/10.1007/s00251-014-0825-y
Shenghe L, Erhui J, Enmei Q, Guozhong W, Kui L (2017) Chitooligosaccharide promotes immune organ development in broiler chickens and reduces serum lipid levels. Histol Histopathol 32(9):951–961. https://doi.org/10.14670/hh-11-860
Liang W, Li H, Zhou H, Wang M, Zhao X, Sun X, Li C, Zhang X (2021) Effects of Taraxacum and Astragalus extracts combined with probiotic Bacillus subtilis and Lactobacillus on Escherichia coli–infected broiler chickens. Poult Sci 100(4):101007. https://doi.org/10.1016/j.psj.2021.01.030
Teo AY, Tan HM (2007) Evaluation of the performance and intestinal gut microflora of broilers fed on corn-soy diets supplemented with Bacillus subtilis PB6 (CloSTAT). Journal of Applied Poultry Research 16(3):296–303. https://doi.org/10.1093/japr/16.3.296
Amat C, Planas JM, Moretó M (1996) Kinetics of hexose uptake by the small and large intestine of the chicken. Am J Physiol 271(4 Pt 2):R1085-1089. https://doi.org/10.1152/ajpregu.1996.271.4.R1085
Liu Y, Zhang J, Wang S, Guo Y, He T, Zhou R (2019) A novel adjuvant “sublancin” enhances immune response in specific pathogen-free broiler chickens inoculated with Newcastle disease vaccine. J Immunol Res 2019:1016567. https://doi.org/10.1155/2019/1016567
Liu X, Cao G, Wang Q, Yao X, Fang B (2015) The effect of Bacillus coagulans-fermented and nonfermented Ginkgo biloba on the immunity status of broiler chickens. J Anim Sci 93(7):3384–3394. https://doi.org/10.2527/jas.2015-8902
Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN (2012) Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 24(6):503–512. https://doi.org/10.1111/j.1365-2982.2012.01921.x
Khan S, Moore RJ, Stanley D, Chousalkar KK (2020) The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl Environ Microbiol 86(13):e00600-00620. https://doi.org/10.1128/AEM.00600-20
Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC (2011) Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 141(5):769–776. https://doi.org/10.3945/jn.110.135657
Zihni C, Mills C, Matter K, Balda MS (2016) Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol 17(9):564–580. https://doi.org/10.1038/nrm.2016.80
Lee SH (2015) Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res 13(1):11–18. https://doi.org/10.5217/ir.2015.13.1.11
Anderson RC, Cookson AL, McNabb WC, Park Z, McCann MJ, Kelly WJ, Roy NC (2010) Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol 10:316. https://doi.org/10.1186/1471-2180-10-316
Tremaroli V, Bäckhed F (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489(7415):242–249. https://doi.org/10.1038/nature11552
Kridtayopas C, Rakangtong C, Bunchasak C, Loongyai W (2019) Effect of prebiotic and synbiotic supplementation in diet on growth performance, small intestinal morphology, stress, and bacterial population under high stocking density condition of broiler chickens. Poult Sci 98(10):4595–4605. https://doi.org/10.3382/ps/pez152
Pan D, Yu Z (2014) Intestinal microbiome of poultry and its interaction with host and diet. Gut microbes 5(1):108–119. https://doi.org/10.4161/gmic.26945
Yan J, Zhou B, Xi Y, Huan H, Li M, Yu J, Zhu H, Dai Z, Ying S, Zhou W, Shi Z (2019) Fermented feed regulates growth performance and the cecal microbiota community in geese. Poult Sci 98(10):4673–4684. https://doi.org/10.3382/ps/pez169
Wei S, Morrison M, Yu Z (2013) Bacterial census of poultry intestinal microbiome. Poult Sci 92(3):671–683. https://doi.org/10.3382/ps.2012-02822
Brulc JM, Antonopoulos DA, Miller ME, Wilson MK, Yannarell AC, Dinsdale EA, Edwards RE, Frank ED, Emerson JB, Wacklin P, Coutinho PM, Henrissat B, Nelson KE, White BA (2009) Gene-centric metagenomics of the fiber-adherent bovine rumen microbiome reveals forage specific glycoside hydrolases. Proc Natl Acad Sci U S A 106(6):1948–1953. https://doi.org/10.1073/pnas.0806191105
Wang L, Hatem A, Catalyurek UV, Morrison M, Yu Z (2013) Metagenomic insights into the carbohydrate-active enzymes carried by the microorganisms adhering to solid digesta in the rumen of cows. PLoS One 8(11):e78507. https://doi.org/10.1371/journal.pone.0078507
Spence C, Wells WG, Smith CJ (2006) Characterization of the primary starch utilization operon in the obligate anaerobe Bacteroides fragilis: Regulation by carbon source and oxygen. J Bacteriol 188(13):4663–4672. https://doi.org/10.1128/jb.00125-06
Magrone T, Jirillo E (2013) The interplay between the gut immune system and microbiota in health and disease: nutraceutical intervention for restoring intestinal homeostasis. Curr Pharm Des 19(7):1329–1342. https://doi.org/10.2174/138161213804805793
Nami Y, Vaseghi Bakhshayesh R, Mohammadzadeh Jalaly H, Lotfi H, Eslami S, Hejazi MA (2019) Probiotic properties of Enterococcus isolated from artisanal dairy products. Front Microbiol 10:300. https://doi.org/10.3389/fmicb.2019.00300
Ben Braïek O, Smaoui S (2019) Enterococci: between emerging pathogens and potential probiotics. BioMed Res Int 2019:5938210. https://doi.org/10.1155/2019/5938210
Turroni F, Ventura M, Buttó LF, Duranti S, O’Toole PW, Motherway MO, van Sinderen D (2014) Molecular dialogue between the human gut microbiota and the host: a Lactobacillus and Bifidobacterium perspective. Cell Mol Life Sci 71(2):183–203. https://doi.org/10.1007/s00018-013-1318-0
Onrust L, Ducatelle R, Van Driessche K, De Maesschalck C, Vermeulen K, Haesebrouck F, Eeckhaut V, Van Immerseel F (2015) Steering endogenous butyrate production in the intestinal tract of broilers as a tool to improve gut health. Front Vet Sci 2:75. https://doi.org/10.3389/fvets.2015.00075
Acknowledgements
We are extremely grateful to the Central Laboratory of the College of Veterinary Medicine and Animal Microecology Institute of Sichuan Agricultural University for providing assistance and support during the whole process of the experiment.
Funding
Financial supports were obtained from the Project of Science and Technology Innovation Research Team in the University of Sichuan Province (grant no. KM406183.1), the Scientific Research Fund Project of Chengdu Agricultural College (grant no. 20ZR102), and the Scientific Research Interest Cultivation Program of Sichuan Agricultural University (grant no. 2020167).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures were performed according to the guidelines for the care and use of laboratory animals approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University (approval number: DY2018203012) before the start of the experiment.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors would like to state that the manuscript has not been published and is not under consideration for publication elsewhere.
Dan Xiao, Zhenhua Wang, and Xixi Dai were joint first authors and contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xiao, D., Wang, Z., Dai, X. et al. Effects of Bacillus methylotrophicus SY200 Supplementation on Growth Performance, Antioxidant Status, Intestinal Morphology, and Immune Function in Broiler Chickens. Probiotics & Antimicro. Prot. 15, 925–940 (2023). https://doi.org/10.1007/s12602-022-09924-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-09924-6