Abstract
Purpose
During cancer surgery, prostaglandin-mediated inflammation may promote and activate micrometastatic disease with a consequent increase in long-term cancer recurrence. Cyclooxygenase-2 inhibitors, known to have anti-proliferative properties, may offset such perioperative perturbation. We investigated the effectiveness of these agents to minimize inflammatory changes during cancer surgery.
Methods
Following ethics approval, 32 patients who were to undergo major intracavity cancer surgery were enrolled in this prospective, randomized, clinical trial. The treatment group received 400 mg celecoxib preoperatively followed by five 200 mg 12-hourly doses. The control group received no anti-inflammatory agents. Inflammatory and immunomodulatory end points were measured serially. The primary end points were the measured plasma and urinary prostaglandin E metabolite (PGEM) levels 48 hours following surgery. Secondary endpoints included interleukin levels, leucocyte profile, and clinical end points.
Results
No differences in the 48-hr plasma or urinary PGEM levels were observed between the celecoxib and control groups. Linear mixed modeling, used to accommodate differences in baseline PGEM levels, showed that celecoxib (cf. control) administration lowered plasma PGEM over the entire 48-hr period following surgery (β-coefficient = −0.38 pg.ml−1; 95% confidence interval: −0.69 to −0.06; P = 0.021). Celecoxib administration also lowered postoperative pain scores.
Discussion
Standard dosing of the cyclooxygenase-2 inhibitor celecoxib slightly reduced perioperative cyclooxygenase activity during cancer surgery. Given cyclooxygenase’s role in cancer pathways, we recommend dose-finding studies be undertaken before prospective clinical trials are conducted testing the currently unsubstantiated hypothesis that perioperative anti-inflammatory administration improves long-term cancer outcomes. This trial was registered at: Australian New Zealand Clinical Trial Registry: ACTRN12615000041550; www.anzctr.org.au
Résumé
Objectif
Pendant les chirurgies du cancer, l’inflammation médiée par les prostaglandines pourrait favoriser et activer une maladie micrométastatique avec une augmentation conséquente de la récurrence du cancer à long terme. Les inhibiteurs de la cyclo-oxygénase-2, dont on connaît les propriétés antiprolifératives, pourraient compenser une telle perturbation périopératoire. Nous avons étudié l’efficacité de ces agents pour minimiser les changements inflammatoires pendant les chirurgies du cancer.
Méthode
Après avoir obtenu le consentement du Comité d’éthique, 32 patients devant subir une chirurgie ouverte majeure pour un cancer ont été enrôlés dans cette étude clinique prospective et randomisée. Le groupe traitement a reçu 400 mg de célécoxib avant l’opération, puis cinq doses de 200 mg aux 12 heures. Le groupe témoin n’a reçu aucun agent anti-inflammatoire. Les critères d’évaluation d’inflammation et d’immunomodulation ont été mesurés en série. Les critères d’évaluation principaux étaient les taux de métabolites des prostaglandines E (PGEM) mesurés dans le plasma et dans l’urine 48 h après la chirurgie. Les critères d’évaluation secondaires comprenaient les taux d’interleukine, le profil leucocytaire ainsi que des critères d’évaluation cliniques.
Résultats
Aucune différence n’a été observée dans les taux de PGEM dans le plasma ou l’urine à 48 h entre le groupe célécoxib et le groupe témoin. Un modèle linéaire mixte, utilisé pour tenir compte des différences dans les taux de base de PGEM, a démontré que l’administration de célécoxib réduisait les PGEM dans le plasma tout au long de la période de 48 h suivant la chirurgie (coefficient β = −0,38; intervalle de confiance 95 % : −0,69 à −0,06; P = 0,021). L’administration de célécoxib a également réduit les scores de douleur postopératoires.
Discussion
Une posologie standard de l’inhibiteur de cyclo-oxygénase 2 qu’est le célécoxib a légèrement réduit l’activité périopératoire de la cyclo-oxygénase pendant une chirurgie du cancer. Étant donné le rôle de la cyclo-oxygénase dans les voies de développement du cancer, nous recommandons de tester l’hypothèse, actuellement non vérifiée, selon laquelle l’administration périopératoire d’anti-inflammatoires améliorerait les pronostics oncologiques à long terme, avant de réaliser des études cliniques prospectives. Cette étude est enregistrée au : Registre australien et néozélandais des études cliniques : ACTRN12615000041550; www.anzctr.org.au
Similar content being viewed by others
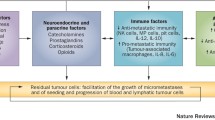
Perioperative surgical stress up-regulates patients’ adrenergic-inflammatory pathways. These changes are linked with cancer progression and are potentially mediated by increasing the susceptibility for activation or initiation of micrometastatic disease.1–4 This hypothesis is supported by animal studies5 and retrospective clinical studies that associated interventions that reduced the perioperative stress response with improved cancer outcomes, including spinal anesthesia,6 epidural analgesia,7 non-steroidal anti-inflammatory drugs (NSAIDs),8 and beta-blockade.9
Perioperative inflammation increases cyclooxygenase (COX) activity, thereby elevating prostaglandin (PG) and cytokine levels.10,11 Prostaglandins promote cancer processes12 by facilitating tumour growth,13 tumour invasion,14 and lymphatic-mediated metastasis.15 High COX-2 expression in lung,16 breast,17 colon,18 and cervical14 tumours is associated with poor survival. As a correlation exists between surgical-site and systemic prostaglandin E (PGE) levels,19 elevated plasma PGE may be a useful biomarker throughout the perioperative period of cancer surgery as an indicator of adequate blockade of inflammatory processes. Prostaglandin E metabolite (PGEM) - a promising cancer biomarker of treatment response and recurrence risk20 - is more easily analyzed than the rapidly metabolized plasma PGE2, but is untested as a marker of perioperative inflammatory response in the cancer surgery setting.20,21
Observational studies have reported that perioperatively administered NSAIDs are associated with improved disease-free survival.8,22 Potential mechanisms include prevention of micrometastatic disease activation via reduced inflammation.2
In animals, COX-2 inhibitors (in contrast to non-selective COX inhibitors) prevent cancer progression.5,23 Cyclooxygenase-2 inhibitors’ capacity to mitigate surgery-induced inflammation and specific cancer markers of immunosuppression requires further study. The present trial undertook serial measurement of inflammatory markers (plasma PGEM [pPGEM] and urinary PGEM [uPGEM]) during the perioperative period of cancer surgery. Our primary hypothesis was that the selective COX-2 inhibitor celecoxib would suppress an anticipated perioperative increase in these inflammatory markers. The primary endpoints were pPGEM and uPGEM levels 48 hours following surgery commencement, capturing the period of peak perioperative inflammatory response.11 Secondary end points included plasma cytokine concentrations, leucocyte profile changes, and clinical end points.
Methods
This institutionally approved (13/06, Peter MacCallum Cancer Centre Human Research Ethics Committee, St. Andrews Place, Melbourne, Australia, January 2015), prospectively registered, proof-of-concept mechanistic study was conducted with data collection during January to August 2015 at the Peter MacCallum Cancer Centre, Melbourne, Australia. Following attainment of written informed consent, patients were randomized (1:1) to celecoxib or control (no NSAID) groups on the day of surgery using the closed double envelope technique (prepared independently and administered by the institution’s Biostatistics and Clinical Trials Centre), and were unblinded throughout the study.
Study criteria and recruitment
Patients more than 18 years of age with a cancer diagnosis undergoing (non-laparoscopic) intra-abdominal or intra-thoracic surgery were eligible for this study. Exclusion criteria included contraindications to celecoxib, acute inflammatory condition (sepsis, infection), pregnancy or lactation, concurrent use of oral corticosteroid treatment or NSAID/acetylsalicylic acid (within a week prior to study entry), presence of a neuroendocrine tumour, hepatic impairment (aspartate transaminase >240 µ·L−1, alanine transaminase >110 µ·L−1), and/or renal impairment (creatinine >150 μmol·L−1).
Group allocation, dosing, and anesthetic management
A study investigator obtained informed consent and performed patient registration, group allocation (randomization), and the collection of baseline demographic data including exposure to neoadjuvant (preoperative) chemotherapy (12 weeks prior to surgery). Patients underwent routine clinical evaluation including a detailed medical history, clinical examination, standard preoperative blood testing, and electrocardiography.
Patients randomized to celecoxib received a 400 mg oral loading dose one hour prior to surgery and subsequently 200 mg (oral) every 12 hours for five doses postoperatively (a total of six dosing events). The control group received no NSAID throughout the perioperative period. General anesthesia was induced with propofol 1.5-2 mg·kg−1 and rocuronium 0.5-0.75 mg·kg−1. The patients were ventilated with sevoflurane (1.5%-2.2% sevoflurane in a mixture with air/oxygen: 60/40). Opioid administration (fentanyl or morphine) was left to the discretion of the treating anesthesiologist (a non-investigator). Intraoperative steroid medications were avoided. Local anesthesia infiltration was permitted.
Immune mediators measured and laboratory analyses
Venous blood samples were obtained at baseline (preoperative) and six-, 24-, and 48-hr following the commencement of surgery. Samples were analyzed for markers of inflammation: PGEM, cytokine concentrations (interleukin [IL]-6, IL-10), and the IL-6/IL-10 ratio.24 Immunosuppression was analyzed by evaluating the platelet/lymphocyte ratio25 because of its predictive role in cancer-related mortality. Urinary PGEM was analyzed from urine samples obtained at baseline and 48-hr following surgery. All samples were immediately taken to a dedicated on-site laboratory, centrifuged, and the plasma frozen (−80°C) until analyzed.
All laboratory testing was performed on de-identified samples to ensure blinding of scientists to group allocation. The pPGEM and uPGEM were measured using a PGEM enzyme immunoassay kit according to the manufacturer’s instructions (Cayman Chemical, MI, USA). Cytokine analysis was performed using the BD Cytometric Multiplexed Bead-Based Immunoassay (BD Biosciences, San Jose, CA, USA) in accordance with the manufacturer’s instructions. Leucocytes were counted using Cell-Dyn Sapphire (Abbott, IL, USA).
Clinical monitoring and data collection
Clinical review and assessment of patients’ visual analogue scores (VAS) regarding the perception of static and dynamic pain were assessed twice daily prior to each dosing event. Data were compiled pertaining to the Clavien-Dindo severity grading system of postoperative complications and the postoperative morbidity survey (POMS) on postoperative days 5 and 30.26 Data were analyzed at the study’s conclusion.
Statistical analysis
A pre-trial power calculation was based on a previous study of anti-inflammatory use in a non-cancer surgical population that found preoperative celecoxib halved PGE production.19 Using a two-sided significance level of 5%, it was calculated that 30 patients would provide 85% power to detect a 10 pg·mL−1 reduction in pPGEM (from 50 pg·mL−1) at 48 hr after surgery (assuming a standard deviation of 9 pg·mL−1). Hence, we recruited 32 patients.
All data were tested for normality using the Kolmogorov-Smirnov test. Normally distributed data are presented as the mean (SD) and non-normally distributed data as the median (interquartile range). Where the assumption of normal distribution was confirmed, an independent two-sample t-test was used. Otherwise a Mann-Whitney U test was performed. Categorical data were analyzed using the two-tailed Fisher’s exact test. Safety end points and complications (RIFLE-classified kidney injury) are reported as frequency. As this study has a proof-of-concept design, analysis was planned per protocol if patients were compliant with at least five of the six dosing events to which they were randomized.
In addition to the planned analysis of the primary endpoint using a univariate analysis, post hoc linear mixed models using unstructured covariance for repeated measures and random effects were used to assess the effect of celecoxib on PGEM (plasma and urinary concentrations, primary end points), cytokine concentrations, and leucocyte profiles (secondary endpoints) over the entire 48-hr perioperative period after adjusting for the fixed effect of biologically plausible confounding. Predictors that were entered into the fixed effects portion of the model included age, sex, duration of operation, site of operation (abdominal vs thoracic), use of neoadjuvant chemotherapy, beta-blockers, and an interaction term between celecoxib and the use of neoadjuvant chemotherapy. Predictors were then removed in a stepwise fashion starting with the predictors that had the largest P value to obtain a parsimonious model. Covariates were no longer removed if the reduced model was associated with a larger Schwarz’s Bayesian Criterion (BIC). All statistical analyses were conducted using SPSS for Windows (version 23, IBM, Armonk, NY, USA), and P < 0.05 was considered to indicate statistical significance.
Results
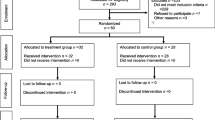
The study was stopped following the recruitment and follow-up (30 days following surgery) of the planned 32 patients. A per-protocol analysis was conducted following exclusion of three patients (Fig. 1). One patient in the control group suffered a pain crisis and was assessed by the treating team as requiring NSAID treatment; one patient’s (celecoxib group) surgical procedure was abandoned after induction of general anesthesia due to disease progression; and one patient’s (celecoxib group) tumour was found to be neuroendocrine in origin after initiation of surgery. The two study groups were similar in baseline characteristics and perioperative parameters (Table 1).
Cyclooxygenase activity and PGEM production
By the 48-hr time point, there were no significant differences between the celecoxib and control groups regarding the unadjusted mean pPGEM concentration (21.5 vs 22.6 pg·mL−1; P = 0.65, Fig. 2) or the uPGEM concentration (8.4 vs 9.9 pg·mL−1; P = 0.66) (Table 2). The six-hour mean pPGEM concentration, however, was lower in the celecoxib group (22.7 vs 28.8 pg·mL−1; P = 0.02).
A linear mixed model was utilized to account for baseline differences in the PGEM concentrations for the observations at each time point and to examine the effect of celecoxib on pPGEM over the entire 48-hr perioperative period. It demonstrated that celecoxib lowered the pPGEM concentrations over the 48-hr perioperative period compared with that of the control group (β-coefficient = −0.38 pg·mL−1; 95% confidence interval [CI]: −0.69 to −0.06 pg·mL−1; P = 0.02, Table 3).
Factors including age, sex, surgical site, and duration of the operation were not significantly associated with changes in pPGEM concentrations and so were removed during the modeling process. Further removal of neoadjuvant chemotherapy (P = 0.62) from the final linear mixed model resulted in an inferior model according to the information criterion (BIC = 320), but use of celecoxib remained significantly associated with reduced pPGEM concentration compared with that of the control group. In a separate model, no covariates (including celecoxib) were associated with changes in uPGEM concentration.
Cytokines and immune cell profile
Markers of an inflammatory response to surgery (thrombocytosis, lymphopenia, elevated IL-6/IL-10 ratio) were elevated in the control group (cf. celecoxib) at specific time points (Table 2). During linear mixed model testing, however, celecoxib prevented only the thrombocytosis that was observed in the control group (β-coefficient = 35.4 × 109·L−1; 95% CI: −70 to −0.3 × 109·L−1; P = 0.049), after adjusting for use of neoadjuvant chemotherapy (P = 0.11). The use of celecoxib was not significantly associated with changes in IL-6 (P = 0.669), IL-10 (P = 0.42), white blood cell count (P = 0.62), lymphocyte count (P = 0.94), or the platelet/lymphocyte ratio (P = 0.07).
Twelve patients (six controls, six with celecoxib treatment) were given neoadjuvant chemotherapy, which was associated with a lower lymphocyte count (β-coefficient = −0.53 × 109·L−1; 95% CI: −0.92 to −0.15 × 109·L−1; P = 0.01) and a higher platelet/lymphocyte ratio (β-coefficient = 126; 95% CI: 83 to 169; P = 0.001).
Pain and clinical outcomes
Celecoxib resulted in significantly lower static (rest) and dynamic (movement) VAS pain scores at 48 hr following surgery (Fig. 3). The POMS at postoperative days 5 and 30 (POMS-5 and POMS-30, respectively) did not differ between the two groups (Table 1). One control patient developed sepsis necessitating inotropic supportive care following surgery. No postoperative mortality occurred.
Discussion
When compared with no NSAID administration, perioperative dosing of the selective COX-2 inhibitor celecoxib resulted in a slight reduction (pPGEM) in COX activity throughout the first 48 hr of the perioperative period following cancer surgery, although there was no difference between the two groups regarding the uPGEM concentration. Most of the difference in pPGEM between the groups was seen during the early perioperative phase. The clinical significance of such a small difference in a surrogate marker of inflammation is currently unknown.
The importance of prostaglandins in cancer pathophysiology13,15 and progression14,16 has led to the use of PGEM as a cancer biomarker.27,28 As unregulated COX activity during cancer surgery has been hypothesized to affect patients’ long-term cancer outcomes,8 our study has shown that pPGEM can be measured and is slightly inhibited by celecoxib perioperatively.
The role of celecoxib as an adjunctive aid to chemotherapy has been demonstrated in non-surgical settings29 and was found to be most efficacious for obtaining complete PGEM suppression at high (800-1600 mg daily)30,31 dosage.32 In the present study, we observed only modest suppression of COX activity by celecoxib. This may have been due to neoadjuvant chemotherapy’s dysregulation of the post-transcriptional modification of COX-2 messenger RNA,33 limiting celecoxib’s effectiveness.20 Alternatively, celecoxib either does not achieve profound PGEM suppression perioperatively or can do so only at a higher dosage.
To characterize celecoxib’s effectiveness as a perioperative anti-inflammatory agent more broadly, inflammatory (IL-6) and ‘anti-inflammatory’ (IL-10) cytokine concentrations were measured as secondary end points.34 These cytokines are relevant to cancer processes through their regulation of T-helper cell differentiation and natural killer cell activity.2 An elevated IL-6/IL-10 ratio is associated with excessive inflammatory response24 and cancer progression.35 Although celecoxib prevented an elevated IL-6/IL-10 ratio at 24 hr, it did not correlate with changes in the clinical end points (although our study was underpowered to assess such an association).
Several studies have reported that altered perioperative immune function (lymphopenia, elevated platelet/lymphocyte ratio) is associated with poor cancer survival.25,36 Our study found that celecoxib prevented thrombocytosis (an acute-phase response), lymphopenia, and hence an elevation of the platelet/lymphocyte ratio during the early perioperative period. Of interest for future study is whether celecoxib preserves lymphocyte subpopulations (e.g. natural killer cells), which are vital anti-cancer effector cells.
As our study’s primary end points were biochemical surrogates, a considered decision was made not to employ a placebo-based control. However, the lack of patient blinding confounds the interpretation of celecoxib’s improvement in the VAS scores – a secondary endpoint. In this small proof-of-concept study, the risk of a type I statistical error remains high, and one should be cautioned not to over-interpret the study’s findings. Importantly, although celecoxib slightly reduced a COX biomarker (pPGEM) and was associated with improved VAS scores, the study was not powered to detect any improvements in clinical end points, particularly in reference to cancer outcomes.
In summary, this study found that standard dosing of the selective COX-2 anti-inflammatory drug celecoxib produced slight perioperative inhibition of COX as assessed by pPGEM levels. Thus, pPGEM may become a useful biomarker for determining effective COX blockade, particularly during the perioperative period following cancer surgery where anti-inflammatory techniques are postulated to improve patients’ cancer outcomes. Currently, there is no strong evidence that an anti-inflammatory perioperative regimen could have an impact on long-term cancer outcomes. We suggest, then, that before large-scale trials investigating a potential benefit of perioperative NSAIDs on improved long-term cancer outcomes are contemplated, future studies first need to consider the optimal perioperative dosing strategy for NSAIDs.
References
Wang D, DuBois RN. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 2015; 36: 1085-93.
Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol 2015; 12: 213-26.
Torrance HD, Pearse RM, O’Dwyer MJ. Does major surgery induce immune suppression and increase the risk of postoperative infection? Curr Opin Anaesthesiol 2016; 29: 376-83.
Tohme S, Yazdani HO, Al-Khafaji AB, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res 2016; 76: 1367-80.
Glasner A, Avraham R, Rosenne E, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol 2010; 184: 2449-57.
Gottschalk A, Brodner G, Van Aken HK, Ellger B, Althaus S, Schulze HJ. Can regional anaesthesia for lymph-node dissection improve the prognosis in malignant melanoma? Br J Anaesth 2012; 109: 253-9.
Hiller JG, Hacking MB, Link EK, Wessels KL, Riedel BJ. Perioperative epidural analgesia reduces cancer recurrence after gastro-oesophageal surgery. Acta Anaesthesiol Scand 2014; 58: 281-90.
Forget P, Vandenhende J, Berliere M, et al. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg 2010; 110: 1630-5.
Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 2011; 29: 2645-52.
Moselli NM, Baricocchi E, Ribero D, Sottile A, Suita L, Debernardi F. Intraoperative epidural analgesia prevents the early proinflammatory response to surgical trauma. Results from a prospective randomized clinical trial of intraoperative epidural versus general analgesia. Ann Surg Oncol 2011; 18: 2722-31.
Buvanendran A, Kroin JS, Berger RA, et al. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology 2006; 104: 403-10.
Wang D, DuBois RN. Eicosanoids and cancer. Nat Rev Cancer 2010; 10: 181-93.
Hansen-Petrik MB, McEntee MF, Jull B, Shi H, Zemel MB, Whelan J. Prostaglandin E(2) protects intestinal tumors from nonsteroidal anti-inflammatory drug-induced regression in Apc(Min/+) mice. Cancer Res 2002; 62: 403-8.
Ferrandina G, Lauriola L, Distefano MG, et al. Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. J Clin Oncol 2002; 20: 973-81.
Karnezis T, Shayan R, Caesar C, et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 2012; 21: 181-95.
Wu YC, Su LJ, Wang HW, et al. Co-overexpression of cyclooxygenase-2 and microsomal prostaglandin E synthase-1 adversely affects the postoperative survival in non-small cell lung cancer. J Thorac Oncol 2010; 5: 1167-74.
Costa C, Soares R, Reis-Filho JS, Leitao D, Amendoeira I, Schmitt FC. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol 2002; 55: 429-34.
Ogino S, Kirkner GJ, Nosho K, et al. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin Cancer Res 2008; 14: 8221-7.
Zhao H, Feng Y, Wang Y, Yang B, Xing Z. Comparison of different loading dose of celecoxib on postoperative anti-inflammation and analgesia in patients undergoing endoscopic nasal surgery-200 mg is equivalent to 400 mg. Pain Med 2011; 12: 1267-75.
Wang D, DuBois RN. Urinary PGE-M: a promising cancer biomarker. Cancer Prev Res (Phila) 2013; 6: 507-10.
Seyberth HW, Sweetman BJ, Frolich JC, Oates JA. Quantifications of the major urinary metabolite of the E prostaglandins by mass spectrometry: evaluation of the method’s application to clinical studies. Prostaglandins 1976; 11: 381-97.
Liu JF, Jamieson GG, Wu TC, Zhu GJ, Drew PA. A preliminary study on the postoperative survival of patients given aspirin after resection for squamous cell carcinoma of the esophagus or adenocarcinoma of the cardia. Ann Surg Oncol 2009; 16: 1397-402.
Backhus LM, Sievers E, Lin GY, et al. Perioperative cyclooxygenase 2 inhibition to reduce tumor cell adhesion and metastatic potential of circulating tumor cells in non-small cell lung cancer. J Thorac Cardiovasc Surg 2006; 132: 297-303.
Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med 1999; 27: 1262-4.
Goh BK, Chok AY, Allen JC Jr, et al. Blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are independent prognostic factors for surgically resected gastrointestinal stromal tumors. Surgery 2016; 159: 1146-56.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205-13.
Cai Q, Gao YT, Chow WH, et al. Prospective study of urinary prostaglandin E2 metabolite and colorectal cancer risk. J Clin Oncol 2006; 24: 5010-6.
Dong LM, Shu XO, Gao YT, et al. Urinary prostaglandin E2 metabolite and gastric cancer risk in the Shanghai women’s health study. Cancer Epidemiol Biomarkers Prev 2009; 18: 3075-8.
Mao JT, Fishbein MC, Adams B, et al. Celecoxib decreases Ki-67 proliferative index in active smokers. Clin Cancer Res 2006; 12: 314-20.
Kurtova AV, Xiao J, Mo Q, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 2015; 517: 209-13.
Altorki NK, Port JL, Zhang F, et al. Chemotherapy induces the expression of cyclooxygenase-2 in non-small cell lung cancer. Clin Cancer Res 2005; 11: 4191-7.
Reckamp KL, Krysan K, Morrow JD, et al. A phase I trial to determine the optimal biological dose of celecoxib when combined with erlotinib in advanced non-small cell lung cancer. Clin Cancer Res 2006; 12(11 Pt 1): 3381-8.
Dixon DA. Dysregulated post-transcriptional control of COX-2 gene expression in cancer. Curr Pharm Des 2004; 10: 635-46.
Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med 2001; 163: 316-21.
Cai T, Mazzoli S, Meacci F, et al. Interleukin-6/10 ratio as a prognostic marker of recurrence in patients with intermediate risk urothelial bladder carcinoma. J Urol 2007; 178: 1906-11.
Que Y, Qiu H, Li Y, et al. Preoperative platelet-lymphocyte ratio is superior to neutrophil-lymphocyte ratio as a prognostic factor for soft-tissue sarcoma. BMC Cancer 2015; 15: 648.
Acknowledgements
The authors thank Professor Paul Myles for his advice regarding the trial’s conduct and manuscript preparation.
Disclosures
No external funding is declared. No conflicting commercial or non-commercial affiliations and no competing interests are declared.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Associate Editor, Canadian Journal of Anesthesia.
Author contributions
Jonathan G. Hiller, Shienny Sampurno, Rosemary Millen, Niketh Kuruvilla, Kwok M. Ho, Rob Ramsay, and Bernhard Riedel made substantial contributions to the study design, data acquisition, analysis and interpretation, and manuscript drafting and provided final approval. Jonathan G. Hiller, Niketh Kuruvilla, and Bernhard Riedel were responsible for the majority of patient recruitment. Jonathan G. Hiller, Shienny Sampurno, Rosemary Millen, and Rob Ramsay were primarily responsible for the processing and analysis of blood samples pertaining to the primary and secondary end points. Jonathan G. Hiller, Niketh Kuruvilla, and Bernhard Riedel were primarily responsible for acquiring clinical perioperative data pertaining to study end points. Kwok M. Ho was primarily responsible for data analysis. The first draft of the manuscript was prepared by Jonathan G. Hiller and subsequently reviewed and edited by all co-authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hiller, J.G., Sampurno, S., Millen, R. et al. Impact of celecoxib on inflammation during cancer surgery: a randomized clinical trial. Can J Anesth/J Can Anesth 64, 497–505 (2017). https://doi.org/10.1007/s12630-017-0818-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-017-0818-z