Abstract
Purpose
To perform a narrative review of the current trials examining the use of perioperative ultrasound to diagnose common issues related to the heart, lungs, stomach, and airway.
Method
A review of the current literature was conducted in June 2017 on all trials involving ultrasound, including both surface and transesophageal ultrasound, in the perioperative period. The search included the terms ‘ultrasonography’, ‘perioperative care’, ‘point-of-care’, and ‘bedside’. Trials were limited to human subjects with no language or time restrictions being applied. The results were then collected and a narrative review was completed with the available information.
Results
In total 1,176 reports of original investigation or systematic reviews were collected and reviewed. Of those 1,176 reports and reviews, a total of 80 original articles met the inclusion criteria for this review. Topics were broadly defined based on common themes emerging from the literature including cardiac disease, lung pathology (pneumothorax, pleural effusion, pulmonary edema, and pulmonary consolidation), volume and contents of the stomach, confirmation of endotracheal tube position, confirmation of lung isolation, and the application of ultrasound for guiding cricothyroidotomy. Where possible, the sensitivity and specificity of the trials are presented. Few trials reported on patient outcomes, although several discussed provider outcomes such as a change in anesthesia practice. In addition, trials reporting outcomes, although few in number, were included.
Conclusion
Perioperative point-of-care ultrasound is a useful method for the diagnosis of many important perioperative conditions. The impact of this diagnostic approach on patient outcomes however remains to be determined.
Résumé
Objectif
Proposer une étude narrative des essais cliniques publiés sur l’utilisation de l’échographie en période périopératoire pour le diagnostic des fréquents en rapport avec le cœur, les poumons, l’estomac et les voies respiratoires supérieures.
Méthode
Une étude des publications a été menée en juin 2017 sur toutes les études impliquant l’échographie, à la fois de surface ou transœsophagienne, au cours de la période périopératoire. La recherche a inclus les termes échographie, soins périopératoires, soins au point d’intervention et au chevet des patients. Les essais ont été limités aux sujets humains sans restriction de langue ou de temps. Les résultats ont été colligés et une synthèse narrative a été effectuée à partir des renseignements disponibles.
Résultats
Au total, 1 176 études systématiques ou comptes rendus d’études originales ont été rassemblés et analysés. Parmi ces 1 176 études systématiques et comptes rendus, un total de 80 articles ont satisfait les critères d’inclusion de cette synthèse. Les sujets étaient définis de façon large sur des thèmes fréquents provenant de la littérature, notamment maladie cardiaque, maladies pulmonaires (pneumothorax, épanchement pleural, œdème pulmonaire et consolidation pulmonaire), volume et contenu de l’estomac, confirmation de la position d’un tube endotrachéal, confirmation de l’exclusion d’un poumon, et utilisation de l’échographie pour guider une cricothyroidotomie. La sensibilité et spécificité des études sont présentées quand cela est possible. Peu d’essais ont indiqué l’évolution des patients, bien que plusieurs aient discuté des répercussions pour le praticien, telles que les changements de pratique pour l’anesthésie. De plus, des essais indiquant des résultats, quoique peu nombreux, ont été inclus.
Conclusion
L’échographie au point d’intervention en période périopératoire est une méthode utile au diagnostic de nombreuses affections périopératoires importantes. Néanmoins, l’impact de cette démarche diagnostique sur l’évolution des patients reste à déterminer.
Similar content being viewed by others
The use of bedside ultrasound outside of radiology or cardiology departments began with the use of transesophageal echocardiography in the cardiac operating rooms. At roughly the same time, the benefits of portable ultrasound began to be realized in both the emergency room and critical care settings. Outside of the operating room, the use of transthoracic imaging became the predominant approach.
In the emergency departments, questions regarding the severity of blunt force trauma to the torso have been a major concern for physicians, which led to the development of focused assessment with sonography for trauma (FAST) examination, whereas in the intensive care unit, bedside ultrasound, particularly transthoracic echocardiography, was employed for assessment of hemodynamic instability. Both of these approaches recognize the use of bedside ultrasound to address clinical questions with a limited scope and then aid in both the diagnosis and treatment of the patient in real time.
The use of transthoracic echocardiography in the perioperative setting is now gaining momentum based on the experiences of cardiac anesthesiologists who were familiar with the utilization of cardiac ultrasound in the perioperative setting. Nevertheless, the focus in non-cardiac anesthesia has switched from transesophageal echocardiography to transthoracic ultrasound, using ultrasound to assess not only the heart but also the lungs, stomach (gastric volume), and airway. The use of transthoracic echocardiography has also broadened the time periods in which ultrasound can be employed from purely using ultrasound during the intraoperative period for transesophageal to using transthoracic techniques in the pre-operative, operative, and postoperative periods (Table 1).
While consensus remains to be developed around the role ultrasound should play in the perioperative setting, as well as the questions that can be potentially addressed by its clinical use, the training of the individuals performing the examinations and the requirement for ongoing education, a growing body of evidence is developing on the utility of perioperative bedside ultrasound.
The purpose of this review is to identify the impact of perioperative bedside ultrasound on diagnosis and decision-making when used to assess the heart, lungs, gastric volume, and airway. The review will not consider ultrasound-guided procedures such as regional anesthesia or vascular access and will primarily focus on adult patients.
Search strategy
A literature search was conducted by a medical librarian at our institution (B.M.). The following electronic bibliographic databases were searched: MEDLINE (OVID and PubMed), EMBASE, The Cochrane Library [Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Methodology Register], Health Technology Assessment Database, CINAHL, and Web of Science (science and social science citation index).
The search strategy included the terms ‘ultrasonography’, ‘perioperative care’, ‘point-of-care’, and ‘bedside’. The search terms were adapted for use with other bibliographic databases in combination with database-specific filters for controlled trials when available. There were no language restrictions and no time limits applied to the search strategies. The search was limited to human studies only. The initial search was conducted on 20-23 June 2017.
A hand search and related search of key articles located in the initial search were also subsequently conducted. All abstracts for all identified trials were reviewed and either discarded as not relevant or the full text version was obtained. Review of the full text version resulted in discarding further trials and retaining the rest for inclusion in this review.
Study inclusion criteria were as follows: any study design in any language, adult non-cardiac surgical patients (≥18 yr of age), patients undergoing perioperative ultrasound (either immediately prior to induction or intraoperatively and performed by the anesthesiologist or other designated care provider), and reported findings or diagnoses observed by ultrasound. Where few trials on perioperative studies were available, studies on intensive care patients were included to better inform the scope of potential ultrasound utilization.
Nomenclature
The use of transthoracic and transesophageal echocardiography in the lexicon became commonplace as a method of describing cardiac examination, but provided an ultrasound approach with the organ being examined (the heart) inferred. Even here, the transesophageal examination has frequently been used to assess for pleural effusions and diseases involving the aorta, so strictly speaking it has never been cardiac specific. Ultrasound examination of other organs or body regions typically follows an anatomical approach with abdominal ultrasound, carotid ultrasound, or Doppler ultrasound of the legs being examples. Many terms have been used for ultrasound performed by the treating physician or designate, such as point-of-care ultrasound (POCUS), beside ultrasound, and perioperative ultrasound. The “perioperative period” is also becoming quite indistinct with some anesthesiology departments using ultrasound machines in the pre-admission clinic, thus separating the treating anesthesiologist from the one performing the examination. Many acronyms have been developed to describe the use of point-of-care approaches to certain medical situations such as the FAST examination to rule out free peritoneal fluid, or the FOCUS examination to assess hemodynamic instability. In addition, both transesophageal and transthoracic techniques may be used at the point of care and both probes may be used for lung and other POCUS examinations. In the same way that the anatomical approach for cardiac examinations may affect the diagnostic accuracy, so the probe selection (i.e., linear vs curved, high frequency vs low frequency) for POCUS may have an impact on the diagnosis of various conditions. For the remainder of this review, we will use the term POCUS to refer to ultrasound performed by the anesthesiologist or treating physician performed in close temporal relationship to the procedure or condition being diagnosed. We have used the approach of referring anatomically to the structure being examined and make no assumption about the approach or probe used unless specifically stated.
Results
A total of 1,176 original articles were identified during the search. Following an extensive review of the abstracts, a total of 221 articles were selected for further review, after which 80 reviews in total were identified as relevant for inclusion in to our study (Figure). Of the 80 trials selected for inclusion, seven were reviews or meta-analyses.
Heart
Historically, cardiac ultrasound has played the largest role in POCUS in the operating room environment. As a clinical tool, it has been used specifically to identify potential causes of hypotension, low cardiac output, or heart failure. Point-of-care ultrasound is available in the cardiac operating rooms in the form of transesophageal echocardiography; however, this review will not deal with that specific use.
The widespread adoption of transesophageal echocardiography in cardiac surgery has led to adoption of transesophageal echocardiography in the non-cardiac operating rooms. While early studies of perioperative cardiac ultrasound used transesophageal echocardiography, a mixture of both transthoracic and transesophageal modalities have more recently been employed.
The patient populations examined fell into two broad groups. The first are patients who are at high risk prior to their operation and the ultrasound examination is used as a screening tool prior to induction of anesthesia. This information is used to alter hemodynamic monitoring and management to prevent hypotension or hemodynamic collapse. The second group consists of patients who are suffering unexplained hypotension or cardiac collapse in the perioperative setting, and ultrasound is used after the event to help diagnosis the cause of the hemodynamic instability.1,2
A recent review by Jasudavisius et al. looked at the use of either screening or rescue ultrasound with the intent of identifying common diagnoses to better inform teaching paradigms.3 The Jasudavisius review identified 14 studies (nine transesophageal, four transthoracic, and one combined) that reported common findings in both the screening and rescue settings. Specifically, a low ejection fraction, aortic valve disease, and mitral valve disease were the top three preoperative diagnoses, while hypovolemia, low ejection fraction, and right ventricular failure were the top three specific intraoperative diagnoses.
Transesophageal echocardiography was utilized in a number of studies examining the impact of ultrasound on planned surgical procedures. Hofer et al. suggested the rate of management change was 47% on intraoperative transesophageal echocardiography examinations performed in non-cardiac surgical patients at risk of hemodynamic instability or myocardial ischemia.4 This was similar to results found by Schulmeyer but higher than a study by Suriani, which had a rate of change in the anesthesia plan of 15%.5,6
Several trials also examined the use of transthoracic ultrasound; for example, Canty et al. showed that peri- and intraoperative POCUS had an impact in 75% of emergency cases and 43% of elective cases.7 In two separate studies by Cowie, the author showed an 80-85% change in care associated with the use of a point-of-care cardiac examination in patients with a clinical indication for a scan.8,9 The primary reason for ultrasound examination in Cowie’s studies was an undifferentiated systolic murmur, present in roughly 50% of patients.
Canty et al. performed an audit of patients undergoing hip fracture surgery and compared those patients who received bedside echocardiography before surgery with those who did not, and the comparison showed a significant reduction in mortality at 12 months after surgery.10 The highest level of evidence to date is from a recent study by Canty et al.,11 who reported a randomized pilot study of 100 participants undergoing surgery from hip fracture who then received bedside transthoracic echocardiography prior to surgery and a control group who did not receive preoperative transthoracic echocardiography assessment. The incidence of a combined primary outcome of any death, acute kidney injury, non-fatal myocardial infarction, cerebrovascular accident, pulmonary embolism, or cardiopulmonary arrest within 30 days of surgery was 14% in the bedside echocardiography group and 24% in the control group. In the transthoracic echocardiography group, there was a change in diagnosis in 26 (53%) and a change in management in 17 (35%); in nine cases, imaging identified significant unexpected pathology prompting stepped-up treatment, and in eight cases, echocardiography excluded significant suspected pathology, prompting stepped-down treatment. Unsuspected cardiac pathology led to increased use of invasive monitoring, use of vasopressor or inotrope infusions, reduced fluid infusions, and disposition after surgery to a high dependency unit or intensive care unit (ICU). Absence of suspected cardiac pathology led to a reduction in planned invasive monitoring, vasoactive medicine, or ICU admissions and increased fluid use if hypovolemia was diagnosed in an otherwise normal heart. Studies in patients requiring intraoperative echocardiography for hemodynamic collapse all suggested that this approach provided useful information to help guide the care of the patient.1,2,12,13
Cardiac echocardiography in patients who clinically warrant an examination appears to have an important impact on patient care, although it is important to acknowledge that there are no large pragmatic trials to evaluate whether the change in management leads to improved outcomes and which patient populations may benefit the most. As skill levels increase, there is likely to be an increase in the use of ultrasound by non-cardiac anesthesiologists to either assess patients at high-risk of intraoperative events or to help guide the therapy of patients who have suffered either severe hemodynamic disturbances or cardiac arrest. A definitive pragmatic randomized trial is recommended.
Lung
The intensive care physicians have taken a strong lead in the use of POCUS for the diagnosis of lung pathology, thereby potentially reducing the cost, inconvenience, and radiation exposure associated with chest x-rays and computed tomographic scans. Ultrasound of the lung can be used to diagnose interstitial edema, atelectasis, consolidation, pleural effusion, and pneumothorax, as shown in Table 2.14,15,16 It is also possible to image the diaphragm to look at the innervation and strength of contraction, which is an approach used in the intensive care unit for ventilator weaning.17
Data for the use of lung ultrasound in the perioperative setting are lacking as current studies have focused primarily on the intensive care setting. Studies specifically from intensive care perspectives have examined the role of lung ultrasound to diagnose common conditions that previously would have required a chest x-ray or a computed tomographic scan.14,15,16 Thus, the main goal of these studies has been to show diagnostic sensitivity and specificity.
Most individual studies suggest diagnostic accuracies > 90% for pleural effusions, 70-90% for consolidation, 60-90% for pulmonary edema, and 90-98% for pneumothorax (Table 2).14,15,16 Ford et al. examined point-of-care lung ultrasound in the perioperative period and compared this method with chest x-ray and clinical assessment to diagnose lung pathology.18 Ultrasound assessment was considered the reference method and was superior to the use of either chest x-ray or clinical assessment alone or combined for the diagnosis of lung disease. In comparison with ultrasound use, sensitivity of the different pathologies ranged from 7-69% for chest x-ray, 7-76% for clinical assessment, and 14-94% when the two methods were combined. Many of the reasons for differences in the sensitivity and specificity of lung ultrasound may lie in the methods used to diagnose a given pathology. For example, the diagnosis of pneumothorax may involve the absence of lung sliding, the A line sign (abolition of lung sliding and exclusive A lines on lung ultrasound), and/or the lung point.19
A recent consensus statement about the use of lung ultrasound in the intensive care unit made strong recommendations for the use of lung ultrasound for ruling in pleural effusion and ruling in pneumothorax, whereas conditional recommendations were given for interstitial and parenchymal lung diseases.20
Lung isolation
Several studies21,22,23,24 have assessed the utility of ultrasound to determine lung isolation during procedures requiring one lung anesthesia or in the assessment for endobronchial intubation when tracheal intubation is desired, and a summary of findings is shown in Table 3.
The identification of lung isolation utilizes lung sliding as the finding to indicate that the lung is being ventilated. Saporito et al.21 compared ultrasound with bronchoscopy and found the two methods equivalent and obtained identical results in all cases except one, in which the patient had no suitable rib window for ultrasound assessment. Alvarez compared clinical assessment (sensitivity 85%, specificity 41%) with ultrasound plus clinical assessment (sensitivity 99%, specificity 53%) and found ultrasound to be superior.22 Ramsingh et al. placed single-lumen endotracheal tubes into the trachea, the right main stem, or the left main stem under flexible bronchscopic guidance and then had blinded assessors determine the tube position,23 showing that ultrasound was superior to auscultation in determining endobronchial intubation with a sensitivity and specificity for ultrasound of 93% and 96% vs auscultation of 66% and 59%. In addition, Sustic et al. compared ultrasound alone vs clinical assessment alone24 and showed that while both methods had a sensitivity of 100%, the specificity was only 22% for clinical assessment, and increased to 50% for ultrasound.
Saporito et al. also examined the costs of the use of ultrasound compared with flexible bronchoscopy. While there were reported cost savings in personnel (i.e., a nurse performed the ultrasound scan while an anesthesiologist was required to perform the bronchoscopy), there were also added costs related to the reprocessing of the bronchoscope.21 Thus, at their clinical institution, Saporito et al. estimated a cost savings of 37 euros per case. The ultrasound examination was also quicker to perform than bronchoscopy.
Ultrasound diagnosis of lung isolation is both rapid and practical in the operating room and may be the technique of choice to confirm lung isolation, but is unlikely to replace the flexible bronchoscope, which can provide visual confirmation of tube position and aid in trouble shooting inadequate lung isolation. Familiarization with ultrasound diagnosis of lung isolation may also aid the anesthesiologist in the diagnosis of main stem intubation with a single-lumen tube in cases where hypoxemia occurs.
Gastric
Gastric volume
Determination of a patient’s fasting status has frequently been done based on history of the last meal/npo status. Nevertheless, gastric emptying may be delayed when there is pain, concomitant opioids, or intra-abdominal pathology. Therefore, the use of ultrasound to assess gastric volume may play an important role in identifying those at increased risk of aspiration.
A systematic review completed in 2014 identified nine papers that correlated ultrasound scanning to gastric volume with correlation coefficients ranging from 0.72-0.91.25 A summary of trials is shown in Table 4. The gold standard method to compare with ultrasound has usually been nasogastric suction; however, gastroscopy and magnetic resonance imaging have also been used. Several of the studies also derived mathematical formulas to convert the antral area into a gastric volume. The cut-off value used by the authors of low vs high risk for aspiration was usually 1.5 mL·kg−1.25,26 In addition to the volume of gastric contents, ultrasound also has the ability to determine whether the stomach contains liquids or particulate matter and thereby allows for additional quantification of the risk from aspiration.25,27,28
It appears that the relationship between the antral area and volume is now generally accepted as most recent literature has turned to evaluation of emptying times of various per os intake materials.29 Work remains to be done as currently several different formulas are used to derive gastric volume based on antral area and there are few data validating aspiration risk against gastric volume.
The study by Bouvet et al. is one of a handful that examined the prevalence of an empty stomach as determined by gastric ultrasound among patients arriving for surgery. Bouvet et al. found a full stomach, defined as the presence of gastric content in the right lateral decubitus and semi-recumbent positions, in 5% of elective patients and 56% of emergent patients.30 A second retrospective study among elective patients found the incidence of full stomach, defined as a calculated stomach volume greater than 1.5 mL·kg−1, was 6.2%, while the incidence of solid content in the stomach was 1.7%.26
Finally, one study examined the impact of gastric ultrasound on the timing of surgery in patients who had consumed solid food or full fluids in a shorter than routine fasting time or in patients who were suspected of having a full stomach following a standard fast. Twenty-seven out of 38 patients had a change in their anesthetic plan as a result of these findings, with 15 patients proceeding with surgery instead of delaying it and 12 patients further delaying surgery.27 This emphasizes the role of perioperative ultrasound as an adjunct to clinical evaluation to better inform the physician.
Airway
Endotracheal placement
Multiple studies have examined the role of ultrasound to identify correct placement of an endotracheal tube; the majority of these studies were performed outside the operating room, usually in the emergency department. To date, two meta-analyses have examined the relative specificity and sensitivity of this approach.31,32 Typically in these studies, the ultrasound probe is placed slightly above the suprasternal notch and evidence for tracheal intubation inferred from a single air-mucosa interface and esophageal intubation inferred by a double air-mucosa interface.31 The results of the findings are presented in Table 5. The primary difference between the inclusion criteria was that Das et al.31 used only clinical trials, while Chou et al.32 included cadaveric studies. Several studies suggest that endotracheal tube depth can be ascertained in pediatric patients, and such an approach may be useful in the future for adults; however, exhaled CO2 remains the gold standard for detection of tracheal intubation as it is both ubiquitous and reliable.
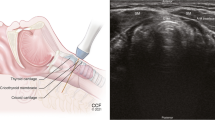
Cricothyroidotomy
Ultrasound may play a useful role in the identification of the puncture site for preforming cricothyroidotomy33,34 in that it is able to identify the relevant thyroid, cricoid, and cricothyroid cartilage by also identifying the appropriate centre of the trachea (medial to lateral). The traditional approach employs palpation to identify structures, which may be inaccurate especially with swelling or trauma to the neck.
There have been several trials and one review looking at the feasibility of ultrasound to guide percutaneous tracheostomy.35,36,37,38,39 Several of these studies have examined the benefits of using ultrasound for percutaneous tracheostomy, and a summary is shown in Table 6.40,41,42,43,44,45,46 Most of the trials were performed on patients requiring elective tracheostomy by either intensivists or otolaryngologists. Ultrasound was used in conjunction with bronchoscopy to help identify the midline instead of palpation and bronchoscopy, though in one study, the ultrasound was used instead of the bronchoscope.41
In general, the aforementioned studies suggest that ultrasound improved the placement accuracy of the guide wire without specifically identifying a reduction in complication rates,41,42 although a few trials did identify reductions in complications.40,43,45 Overall, ultrasound-guided tracheostomy appears to improve the site of tracheal puncture and may improve outcomes.
Conclusion
Perioperative ultrasound involves multiple applications of ultrasound to provide better diagnostic fidelity, which in turn influences clinical management, or to more accurately guide procedures such as vascular access and nerve blocks. The use of perioperative ultrasound has become increasingly popular as more ultrasound machines become available along with an increase in training opportunities for anesthesiologists. The level of training and on-going maintenance of certification within the field of POCUS needs to be more clearly elucidated. The scanning protocols, time points when scanning should occur, and type of patients who benefit need to be explored in more detail. Further large-scale trials are required to determine if the change in diagnosis and management leads to improved patient outcomes.
References
Shillcutt SK, Markin NW, Montzingo CR, Brakke TR. Use of rapid “rescue” perioperative echocardiography to improve outcomes after hemodynamic instability in noncardiac surgical patients. J Cardiothorac Vasc Anesth 2012; 26: 362-70.
Schulmeyer C, Farias J, Rajdl E, de La Maza J, Labbe M. Utility of transesophageal echocardiography during severe hypotension in non-cardiac surgery (Portuguese). Rev Bras Anestesiol 2010; 60: 513-21.
Jasudavisius A, Arellano R, Martin J, McConnell B, Bainbridge D. A systematic review of transthoracic and transesophageal echocardiography in non-cardiac surgery: implications for point-of-care ultrasound education in the operating room. Can J Anesth 2016; 63: 480-7.
Hofer CK, Zollinger A, Rak M, et al. Therapeutic impact of intra-operative transoesophageal echocardiography during noncardiac surgery. Anaesthesia 2004; 59: 3-9.
Schulmeyer MC, Santelices E, Vega R, Schmied S. Impact of intraoperative transesophageal echocardiography during noncardiac surgery. J Cardiothorac Vasc Anesth 2006; 20: 768-71.
Suriani RJ, Neustein S, Shore-Lesserson L, Konstadt S. Intraoperative transesophageal echocardiography during noncardiac surgery. J Cardiothorac Vasc Anesth 1998; 12: 274-80.
Canty DJ, Royse CF. Audit of anaesthetist-performed echocardiography on perioperative management decisions for non-cardiac surgery. Br J Anaesth 2009; 103: 352-8.
Cowie B. Focused cardiovascular ultrasound performed by anesthesiologists in the perioperative period: feasible and alters patient management. J Cardiothorac Vasc Anesth 2009; 23: 450-6.
Cowie B. Three years’ experience of focused cardiovascular ultrasound in the peri-operative period. Anaesthesia 2011; 66: 268-73.
Canty DJ, Royse CF, Kilpatrick D, Bowyer A, Royse AG. The impact on cardiac diagnosis and mortality of focused transthoracic echocardiography in hip fracture surgery patients with increased risk of cardiac disease: a retrospective cohort study. Anaesthesia 2012; 67: 1202-9.
Canty DJ, Royse CF, Kilpatrick D, Williams DL, Royse AG. The impact of pre-operative focused transthoracic echocardiography in emergency non-cardiac surgery patients with known or risk of cardiac disease. Anaesthesia 2012; 67: 714-20.
Lin T, Chen Y, Lu C, Wang M. Use of transoesophageal echocardiography during cardiac arrest in patients undergoing elective non-cardiac surgery. Br J Anaesth 2006; 96: 167-70.
Memtsoudis SG, Rosenberger P, Loffler M, et al. The usefulness of transesophageal echocardiography during intraoperative cardiac arrest in noncardiac surgery. Anesth Analg 2006; 102: 1653-7.
Ashton-Cleary DT. Is thoracic ultrasound a viable alternative to conventional imaging in the critical care setting? Br J Anaesth 2013; 111: 152-60.
Lichtenstein DA. Ultrasound examination of the lungs in the intensive care unit. Pediatr Crit Care Med 2009; 10: 693-8.
Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med 2014; 21: 843-52.
Zambon M, Greco M, Bocchino S, Cabrini L, Beccaria PF, Zangrillo A. Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med 2017; 43: 29-38.
Ford JW, Heiberg J, Brennan AP, et al. A pilot assessment of 3 point-of-care strategies for diagnosis of perioperative lung pathology. Anesth Analg 2017; 124: 734-42.
Lichtenstein DA, Meziere G, Lascols N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med 2005; 33: 1231-8.
Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: general ultrasonography. Crit Care Med 2015; 43: 2479-502.
Saporito A, Lo Piccolo A, Franceschini D, Tomasetti R, Anselmi L. Thoracic ultrasound confirmation of correct lung exclusion before one-lung ventilation during thoracic surgery. J Utrasound 2013; 16: 195-9.
Alvarez-Diaz N, Amador-Garcia I, Fuentes-Hernandez M, Dorta-Guerra R. Comparison between transthoracic lung ultrasound and a clinical method in confirming the position of double-lumen tube in thoracic anaesthesia. A pilot study (Spanish). Rev Esp Anestesiol Reanim 2015; 62: 305-12.
Ramsingh D, Frank E, Haughton R, et al. Auscultation versus point-of-care ultrasound to determine endotracheal versus bronchial intubation: a diagnostic accuracy study. Anesthesiology 2016; 124: 1012-20.
Sustic A, Protic A, Cicvaric T, Zupan Z. The addition of a brief ultrasound examination to clinical assessment increases the ability to confirm placement of double-lumen endotracheal tubes. J Clin Anesth 2010; 22: 246-9.
Van de Putte P, Perlas A. Ultrasound assessment of gastric content and volume. Br J Anaesth 2014; 113: 12-22.
Van de Putte P, Vernieuwe L, Jerjir A, Verschueren L, Tacken M, Perlas A. When fasted is not empty: a retrospective cohort study of gastric content in fasted surgical patients. Br J Anaesth 2017; 118: 363-71.
Alakkad H, Kruisselbrink R, Chin KJ, et al. Point-of-care ultrasound defines gastric content and changes the anesthetic management of elective surgical patients who have not followed fasting instructions: a prospective case series. Can J Anesth 2015; 62: 1188-95.
Perlas A, Chan VW, Lupu CM, Mitsakakis N, Hanbidge A. Ultrasound assessment of gastric content and volume. Anesthesiology 2009; 111: 82-9.
Song IK, Kim HJ, Lee JH, Kim EH, Kim JT, Kim HS. Ultrasound assessment of gastric volume in children after drinking carbohydrate-containing fluids. Br J Anaesth 2016; 116: 513-7.
Bouvet L, Desgranges FP, Aubergy C, et al. Prevalence and factors predictive of full stomach in elective and emergency surgical patients: a prospective cohort study. Br J Anaesth 2017; 118: 372-9.
Das SK, Choupoo NS, Haldar R, Lahkar A. Transtracheal ultrasound for verification of endotracheal tube placement: a systematic review and meta-analysis. Can J Anesth 2015; 62: 413-23.
Chou EH, Dickman E, Tsou PY, et al. Ultrasonography for confirmation of endotracheal tube placement: a systematic review and meta-analysis. Resuscitation 2015; 90: 97-103.
Hiller KN, Karni RJ, Cai C, Holcomb JB, Hagberg CA. Comparing success rates of anesthesia providers versus trauma surgeons in their use of palpation to identify the cricothyroid membrane in female subjects: a prospective observational study. Can J Anesth 2016; 63: 807-17.
Law JA. Deficiencies in locating the cricothyroid membrane by palpation: we can’t and the surgeons can’t, so what now for the emergency surgical airway? Can J Anesth 2016; 63: 791-6.
Guinot PG, Zogheib E, Petiot S, et al. Ultrasound-guided percutaneous tracheostomy in critically ill obese patients. Crit Care 2012; 16: R40.
Rajajee V, Fletcher JJ, Rochlen LR, Jacobs TL. Real-time ultrasound-guided percutaneous dilatational tracheostomy: a feasibility study. Crit Care 2011; 15: R67.
Kollig E, Heydenreich U, Roetman B, Hopf F, Muhr G. Ultrasound and bronchoscopic controlled percutaneous tracheostomy on trauma ICU. Injury 2000; 31: 663-8.
Bonde J, Norgaard N, Antonsen K, Faber T. Implementation of percutaneous dilation tracheotomy-value of preincisional ultrasonic examination? Acta Anaesthesiol Scand 1999; 43: 163-6.
Rudas M, Seppelt I. Safety and efficacy of ultrasonography before and during percutaneous dilatational tracheostomy in adult patients: a systematic review. Crit Care Resusc 2012; 14: 297-301.
Rajajee V, Williamson CA, West BT. Impact of real-time ultrasound guidance on complications of percutaneous dilatational tracheostomy: a propensity score analysis. Crit Care 2015; 19: 198.
Gobatto AL, Besen BA, Tierno PF, et al. Comparison between ultrasound- and bronchoscopy-guided percutaneous dilational tracheostomy in critically ill patients: a retrospective cohort study. J Crit Care 2015; 30: 220.e13-7.
Dinh VA, Farshidpanah S, Lu S, et al. Real-time sonographically guided percutaneous dilatational tracheostomy using a long-axis approach compared to the landmark technique. J Ultrasound Med 2014; 33: 1407-15.
Siddiqui N, Arzola C, Friedman Z, Guerina L, You-Ten KE. Ultrasound improves cricothyrotomy success in cadavers with poorly defined neck anatomy: a randomized control trial. Anesthesiology 2015; 123: 1033-41.
Rudas M, Seppelt I, Herkes R, Hislop R, Rajbhandari D, Weisbrodt L. Traditional landmark versus ultrasound guided tracheal puncture during percutaneous dilatational tracheostomy in adult intensive care patients: a randomised controlled trial. Crit Care 2014; 18: 514.
Yavuz A, Yilmaz M, Goya C, Alimoglu E, Kabaalioglu A. Advantages of US in percutaneous dilatational tracheostomy: randomized controlled trial and review of the literature. Radiology 2014; 273: 927-36.
Sustic A, Kovac D, Zgaljardic Z, Zupan Z, Krstulovic B. Ultrasound-guided percutaneous dilatational tracheostomy: a safe method to avoid cranial misplacement of the tracheostomy tube. Intensive Care Med 2000; 26: 1379-81.
Piccolo AL, Saporito A, Franceschini D, Tomasetti R, Anselmi L. Thoracic ultrasound for confirmation of correct lung exclusion before one lung ventilation in thoracic surgery. Swiss Med Wkly 2014; 144:(Suppl 207): 8S (abstract).
Fujigaki T, Fukusaki M, Nakamura H, Shibata O, Sumikawa K. Quantitative evaluation of gastric contents using ultrasound. J Clin Anesth 1993; 5: 451-5.
Ricci R, Bontempo I, Corazziari E, La Bella A, Torsoli A. Real time ultrasonography of the gastric antrum. Gut 1993; 34: 173-6.
Hveem K, Hausken T, Berstad A. Ultrasonographic assessment of fasting liquid content in the human stomach. Scand J Gastroenterol 1994; 29: 786-9.
Tomomasa T, Tabata M, Nako Y, Kaneko H, Morikawa A. Ultrasonographic assessment of intragastric volume in neonates: factors affecting the relationship between intragastric volume and antral cross-sectional area. Pediatr Radiol 1996; 26: 815-20.
Gilja OH, Detmer PR, Jong JM, et al. Intragastric distribution and gastric emptying assessed by three-dimensional ultrasonography. Gastroenterology 1997; 113: 38-49.
Bouvet L, Miquel A, Chassard D, Boselli E, Allaouchiche B, Benhamou D. Could a single standardized ultrasonographic measurement of antral area be of interest for assessing gastric contents? A preliminary report. Eur J Anaesthesiol 2009; 26: 1015-9.
Bouvet L, Mazoit JX, Chassard D, Allaouchiche B, Boselli E, Benhamou D. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology 2011; 114: 1086-92.
Schmitz A, Thomas S, Melanie F, et al. Ultrasonographic gastric antral area and gastric contents volume in children. Paediatr Anaesth 2012; 22: 144-9.
Perlas A, Mitsakakis N, Liu L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg 2013; 116: 357-63.
Hamada SR, Garcon P, Ronot M, Kerever S, Paugam-Burtz C, Mantz J. Ultrasound assessment of gastric volume in critically ill patients. Intensive Care Med 2014; 40: 965-72.
Spencer AO, Walker AM, Yeung AK, et al. Ultrasound assessment of gastric volume in the fasted pediatric patient undergoing upper gastrointestinal endoscopy: development of a predictive model using endoscopically suctioned volumes. Paediatr Anaesth 2015; 25: 301-8.
Schmitz A, Schmidt AR, Buehler PK, et al. Gastric ultrasound as a preoperative bedside test for residual gastric contents volume in children. Paediatr Anaesth 2016; 26: 1157-64.
Kruisselbrink R, Arzola C, Jackson T, Okrainec A, Chan V, Perlas A. Ultrasound assessment of gastric volume in severely obese individuals: a validation study. Br J Anaesth 2017; 118: 77-82.
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Gregory L. Bryson, Deputy Editor-in-Chief, Canadian Journal of Anesthesia.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bainbridge, D., McConnell, B. & Royse, C. A review of diagnostic accuracy and clinical impact from the focused use of perioperative ultrasound. Can J Anesth/J Can Anesth 65, 371–380 (2018). https://doi.org/10.1007/s12630-018-1067-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-018-1067-5