Abstract
Purpose
Perioperative frailty increases postoperative complications, mortality, and new functional dependence. Despite this, routine perioperative frailty screening is not widespread. We aimed to assess the accuracy of the Clinical Frailty Scale (CFS) as a screening tool prior to anesthesia, and to determine which health domains are affected by frailty.
Methods
In a prospective, single-centre observational study, we enrolled 218 patients aged ≥ 65 yr undergoing elective and emergency surgery. The screening performance of the CFS was compared with the Edmonton Frail Scale, including the effect in individual frailty domains, and outcomes including discharge location and mortality.
Results
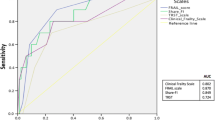
The median [interquartile range] age of the enrolled subjects was 74 [69–80] yr and 24% of the patients were frail. The CFS and Edmonton scales were highly correlated (Spearman correlation coefficient, 0.81; 95% confidence interval [CI], 0.77 to 0.86), and in substantial agreement (kappa coefficient, 0.76; 95% CI, 0.70 to 0.81), with an area under the receiver operating characteristic curve of 0.91 (95% CI, 0.86 to 0.94) indicating excellent discrimination for the CFS in predicting frailty status based on the Edmonton scale. Frail patients had higher 30-day mortality (odds ratio, 5.26; 95% CI, 1.28 to 21.62), and were less likely to be discharged home. Frail patients had poorer health throughout frailty domains, including functional dependence (42% of frail vs 4% of non-frail patients; P < 0.001), malnutrition (48% vs 19%, P < 0.001), and poor physical performance (47% vs 7%, P < 0.001).
Conclusion
The CFS is a valid and accurate tool to screen for perioperative frailty, which encompasses the spectrum of health-related domains.
Résumé
Objectif
La fragilité périopératoire augmente les complications postopératoires, la mortalité et une nouvelle dépendance fonctionnelle. Le dépistage de routine de la fragilité périopératoire n’est cependant pas une pratique répandue. Nous avions pour objectif d’évaluer la précision de l’échelle de mesure de fragilité CFS (pour Clinical Frailty Scale) comme outil de dépistage préanesthésique et de déterminer quels domaines de la santé étaient affectés par la fragilité.
Méthode
Nous avons recruté 218 patients âgés de plus de 65 ans et subissant une chirurgie non urgente ou urgente dans notre étude observationnelle prospective et monocentrique. Les résultats du dépistage de la CFS ont été comparés à l’échelle de fragilité d’Edmonton (Edmonton Frail Scale), y compris en ce qui a trait à l’effet de la fragilité sur les domaines individuels de fragilité et aux résultats tels que la destination au congé et la mortalité.
Résultats
L’âge médian [écart interquartile] des patients recrutés était de 74 [69–80] ans et 24 % des patients étaient fragiles. Les échelles CFS et d’Edmonton avaient une forte corrélation (coefficient de corrélation de Spearman, 0,81; intervalle de confiance [IC] 95 %, 0,77 à 0,86) et étaient en accord substantiel (coefficient kappa, 0,76; IC 95 %, 0,70 à 0,81), avec une surface sous la courbe de fonction d’efficacité de l’observateur de 0,91 (IC 95 %, 0,86 à 0,94), indiquant une discrimination excellente de la CFS pour prédire l’état de fragilité fondé sur l’échelle d’Edmonton. Les patients fragiles souffraient d’une mortalité à 30 jours plus élevée (rapport de cotes, 5,26; IC 95 %, 1,28 à 21,62) et il était moins probable qu’ils reçoivent leur congé de l’hôpital à la maison. Les patients fragiles étaient en moins bonne santé dans tous les domaines de fragilité, notamment en dépendance fonctionnelle (42 % des patients fragiles vs 4 % des patients non fragiles; P < 0,001), en malnutrition (48 % vs 19 %, P < 0,001) et en mauvaise performance physique (47 % vs 7 %, P < 0,001).
Conclusion
L’échelle CFS constitue un outil valable et précis pour dépister la fragilité périopératoire, qui englobe l’éventail des domaines liés à la santé.
Similar content being viewed by others
By 2050, the proportion of the global population aged ≥ 60 yr will more than double, reaching 2.1 billion people.1 In high-income countries, this older cohort already accounts for 30–50% of all surgical procedures, a proportion which is increasing.2,3,4 As a result, the syndrome of increased vulnerability to external stressors known as frailty has emerged as a major area of importance. In older surgical patients, the prevalence of frailty is as high as 40–50%.5 Preoperative frailty also doubles the risk of surgical complications, and leads to increased mortality and length of stay, posing unique challenges for anesthesiologists, surgeons, and health services.6,7 There are two “paradigms” of frailty: the phenotypic model (based on five criteria: exhaustion, weight loss, weakness, and decreased physical activity),8 and the deficit model (which conceptualizes frailty as an accumulation of “health deficits”, or impairments across the spectrum of health).9 The phenotypic model is attractive in its simplicity; however, the deficit model is likely a more comprehensive and multidimensional representation of frailty.
Given this evidence of increased harm, preoperative frailty screening is necessary. Although recommended by the 2010 United Kingdom National Confidential Enquiry into Patient Outcome and Death (NCEPOD) in older surgical patients (which stated that “an agreed means of assessing frailty in the perioperative period should be developed and included in risk assessment”), this is not yet commonplace.10 The challenges are complex and logistical. Comprehensive frailty measurement instruments, such as the 70-item frailty index, take a long time, so are impractical prior to anesthesia.9 Other frailty measurement scales require time, training, and functional testing of patients, constraining widespread uptake.11 Numerous prior studies have investigated more simple frailty instruments in surgical patients to find the optimal scale in the perioperative context. For example, a 2016 North American study of 415 orthopedic patients measured the five-item frailty phenotype scale, showing only moderate concordance with the reference frailty index (weighted kappa = 0.42; 95% confidence interval [CI], 0.36 to 0.49).12 A promising, simplified instrument is the nine-point Clinical Frailty Scale (CFS). This scale enables rapid frailty screening incorporating a range of areas known to be affected by frailty without the need for specific geriatric expertise or functional testing; it was reliable and accurate in other populations.9,13 It also requires considerably less time to administer compared with other multidimensional frailty measurement tools. The CFS has been examined in surgical populations, including a 2018 study of 702 patients undergoing elective non-cardiac surgery, where it was found to be predictive of death, new disability, and institutional discharge.14 Nevertheless, its application in anesthesia and surgery compared with other more validated frailty tools requires further investigation.
Accordingly, we aimed to test the CFS as a frailty screening tool in a cohort of elective and emergency surgical patients. In particular, we hypothesized that the CFS would predict frailty with excellent accuracy and be strongly correlated and in agreement with the Edmonton Frail Scale—a well-researched, well-used, and well-studied multidimensional frailty scale that is too detailed to be applied as a widespread screening tool. We secondarily hypothesized that frailty would affect the full range of health domains in surgical patients and be associated with detrimental postoperative outcomes, including increased mortality and complications.
Methods
This was a prospective, observational cohort study in the perioperative department of the Royal Melbourne Hospital, and formed a complementary, concurrent investigation to our previously published study in 160 critically ill patients.15 The protocol for this frailty research program has previously been published.16 Ethics approval for this study was issued by the Human Research and Ethics Committee of Melbourne Health (20 January 2017, HREC/16/MH/321). Patients aged ≥ 65 yr presenting for either elective or emergency surgery between February 1, 2017 and June 10, 2017 were eligible. Written consent was obtained from either the patient or their next-of-kin if the patient was incapacitated.
Patients who were non-English speaking or presenting for endoscopy procedures were excluded. A convenience sample of patients were enrolled around availability of investigators; only the first operation during the hospitalization was included. Patients were identified from the elective pre-admission anesthetic clinic, or the emergency operating room list. Perioperative data collected included age, sex, height, weight, Charlson comorbidity index, Katz index of independence in daily living,17 residential location prior to admission, P-POSSUM surgical risk score,18 and surgical subspecialty.
Frailty was defined as the state of the patient in the two weeks prior to admission to hospital (elective surgery) or prior to onset of acute illness (emergency surgery) to be consistent with past definitions19; it was preoperatively collected by one of three study investigators. Treating medical teams were blind to patient data collected for this study. Before data collection started, all assessors were trained in the measurement of the CFS by the lead investigator, who has been specifically trained in deriving the CFS score, with ongoing supervision of randomly selected patients throughout the study period. Illness severity, functional ability, and social independence were assessed using the free-text descriptions of these domains contained within the CFS tool. The Edmonton Frail Scale quantifies frailty across nine areas: general health, independence, cognition, social support, medication, nutrition, mood, continence, and function. If the “timed-up-and-go” test was not possible, a Reported Edmonton Frail Scale was conducted, which assesses three areas of reported performance (ability to perform heavy housework, walk up two floors of stairs, walk one kilometre unaided), with a total score of 18 vs 17 for the original scale.20 In the case of abnormal cognition (e.g., delirium or confusion), history from patients’ next-of-kin was obtained; this surrogate history was also used to determine frailty status using the abovementioned definitions if required. Following collection of the Edmonton frailty scale data, the CFS was also measured preoperatively, which is judgement-based around illness severity, functional ability, and social independence.9 The Edmonton frailty scores were subsequently aggregated and the total score assigned after all data were collected.
The primary outcome was agreement and correlation between CFS and Edmonton scales (as described below). Secondary outcomes included 30-day mortality, hospital length of stay, postoperative complications (acute myocardial infarction, cardiac arrest, tracheal reintubation, acute pulmonary edema, deep venous thrombosis, pulmonary embolus, stroke, wound infection, acute kidney injury, unplanned need for reoperation, unplanned admission to the intensive care unit [ICU], all the definitions of which are listed in Electronic Supplementary Material [ESM]); discharge status at postoperative day 30 (i.e,, home, assisted living facility, rehabilitation, other acute hospital, or not discharged); and residential location at six-months postoperatively (i.e., home, acute hospital, assisted-living facility). Outcomes were measured at 30 days postoperatively by investigators not blinded to study data, although the Edmonton frailty scores were aggregated after all data were collected.
Statistical analysis
Continuous data were summarized using mean (standard deviation [SD]) or median [interquartile range (IQR)] in the case of skewed data. Binary and categorical data were summarized as frequencies and percentages. We compared baseline characteristics, health outcomes, and health domains of patients with and without frailty using Chi square or Fisher’s exact tests, two-sample t tests, and Wilcoxon rank-sum tests as appropriate. Univariable and multivariable regression models were also fitted to secondary outcomes, the latter adjusting for age, sex, admission source, Charlson comorbidity index, and emergency/elective surgery. Binary variables with more than one patient were analyzed using Firth logistic regression to obtain the estimated odds ratio and 95% CI.21 The estimated median difference and 95% CI between patients with and without frailty regarding hospital length of stay (days) were obtained from bootstrapped quantile regression with 5,000 replications. Discharge location was analyzed using multinomial logistic regression, yielding the estimated relative risk ratio and 95% CI. Spearman correlation coefficient was used to measure correlation between Edmonton and CFS scales, with inter-rater agreement assessed using Cohen’s kappa between dichotomized (not frail: CFS 1–4, Edmonton 0–7; frail: CFS ≥ 5, Edmonton ≥ 8) and ordinal categories (not frail: CFS 1–3, Edmonton 0–5; vulnerable: CFS 4, Edmonton 6–7; mildly frail: CFS 5, Edmonton 8–9; moderately frail: CFS 6, Edmonton 10–11; severely frail: CFS ≥ 7, Edmonton ≥ 12). The latter were measured via quadratic weighting because higher CFS categories are increasingly important. Kappa coefficients were categorized using the scale of Landis and Koch.22 A Firth logistic regression model was fitted to frailty defined via the Edmonton scale using the ordinal CFS as a predictor to obtain the area under the receiver operating characteristic curve (AUCROC) and its exact binomial 95% CI. The AUCROC was categorized using the general guidelines of Hosmer, Lemeshow, and Sturdivant.23 Sensitivity and specificity were also calculated guided by the optimal cut-off point according to the highest Youden index. We included all enrolled patients in the analyses except in the analysis of residence location at follow-up, which was restricted to patients admitted from home. In addition, we performed separate analyses to examine differences between the whole data set and subgroups according to whether patients were admitted to the ICU or not (and hence included in our previous report).15 No adjustment for multiple testing was performed. STATA 15.1 (College Station, TX, USA) was used for statistical analyses.
Sample size
A sample size of 200 was calculated to have power > 99% (two-sided alpha of 5%) for the Spearman coefficient between the Edmonton and CFS scales to be at least strong (0.80), assuming a correlation of 0.90.24 A sample of 200 participants was also calculated to produce a 95% Clopper-Pearson binomial CI of 15 to 26% around a frailty prevalence estimate of 20%, based on a meta-analysis encompassing more than 8,000 surgical patients. This provided acceptable CIs around likely frailty prevalence for this sample size.6
Results
Two-hundred and eighteen patients were enrolled during the four-month recruitment period, 118 (54%) undergoing elective and 100 (46%) emergency surgery (Fig. 1). Forty-two patients (19%) were admitted to the ICU following surgery, and were included in the previous published analysis.15 Median [IQR] age of patients was 74 [69–80] yr, 99 (45%) of patients were female, with 195 (89%) residing at home prior to surgery. Compared with non-included eligible patients undergoing identical surgical operations in the study period, included patients were of similar age (74 [69–80] yr vs 74 [70–80] yr for non-included patients; P = 0.51), with an increased median [IQR] hospital length of stay (5 [2–13] days vs 4 [1–9] days; P = 0.005). Frailty scales were completed for all included patients; reported physical performance was used for 107 (49%) patients who were unable to perform the timed-up-and-go test. A further 28 (13%) patients had incomplete cognition (clock-drawing) tests because their conscious state had decreased. All other data were complete.
Fifty-two patients (24%; 95% CI, 18 to 30) were measured as frail using the Edmonton scale, compared with 61 (28%; 95% CI, 22 to 34) via the CFS (Fig. 2). The Spearman correlation coefficient comparing the continuous CFS and Edmonton was 0.81 (95% CI, 0.77 to 0.86), indicating a “high” correlation. The kappa coefficient comparing the dichotomized CFS and Edmonton was 0.65 (95% CI, 0.54 to 0.77), and comparing ordinal scales was 0.76 (95% CI, 0.70 to 0.81), both indicating substantial agreement. The AUCROC of the CFS when predicting frailty according to the Edmonton scale was 0.91 (95% CI, 0.86 to 0.94) indicating excellent to outstanding discrimination. A CFS cut-point of “vulnerable” (CFS = 4) captured all but one patient with frailty, as scored by the Edmonton scale, for a sensitivity of 98.1% (95% CI, 89.7 to 100) and specificity of 54.8% (95% CI, 46.9 to 62.5). A CFS cut-point of “mildly frail” (CFS = 5) had the highest Youden index for a sensitivity of 80.8% (95% CI, 67.5 to 90.4) and specificity of 88.6% (95% CI, 82.7 to 93.0). Agreement, correlation, sensitivity, and specificity of the CFS were similar when considering subgroups of patients admitted and not admitted to the ICU (ESM, eTables 1 and 6). Compared with patients without frailty, those scored as frail had higher Charlson comorbidity scores, less functional independence, less likely to be living at home, with higher P-POSSUM scores (Table 1). These associations with frailty persisted when considering the previously unreported subgroup of 176 patients not admitted to ICU (ESM, eTable 2).
After adjusting for potential confounders, mortality at 30 days was higher in patients with frailty using the Edmonton scale (9.6% vs 1.8%; OR, 5.26; 95% CI, 1.28 to 21.62) (Table 2). Any postoperative complications were more common with frailty using the Edmonton scale (OR, 3.67; 95% CI, 1.84 to 7.30), in particular, any unplanned admission to the ICU (OR, 4.54; 95% CI, 1.83 to 11.26), and unplanned return to the operating theatre (OR, 6.20; 95% CI, 2.09 to 18.38). The median [IQR] hospital length of stay was longer in patients with frailty (9.0 [5.0—14.6] vs 3.0 [1.0—9.0] days; mean difference, 2.52; 95% CI, -0.66 to 5.69). When considered via the CFS, patients with frailty were more commonly discharged to assisted living or rehabilitation (23 [38%] frail vs 23 [15%] non-frail) compared with home (29 [48%] frail vs 118 [75%] non-frail; relative risk ratio, 2.66; 95% CI, 1.15 to 6.13). At six-month follow-up, excluding five patients who were lost to follow-up and 33 patients who had died, eight of 41 surviving patients with frailty (20%) were living in an assisted living facility compared with five of 134 surviving patients without frailty (4%), as measured via the CFS. The relative change in the odds of living in an assisted living facility at follow-up, for frail patients compared with non-frail patients (as measured via the CFS) admitted from home, was 1.74-fold (95% CI, 0.37 to 8.16).
Across the spectrum of Edmonton scale domains, patients diagnosed with frailty had significantly worse health; the magnitude of discrepancy in health status was similar whether assessed via the Edmonton and CFS scales (Table 3). Frailty was associated with significantly worse malnutrition (48% vs 19% patients; P = 0.001), medication usage (92% vs 45% of patients taking five or more medications; P < 0.001), higher functional dependence (42% vs 4% of patients; P < 0.001), and lower physical performance (47% vs 7% of patients requiring > 20 sec or unable to perform a timed-up-and-go test, P < 0.001). Patients with frailty also had considerably more hospital admissions in the year prior to surgery compared with patients without frailty (P < 0.001). When separately considering subgroups of patients admitted and not admitted to ICU, demographics were similar apart from higher median [IQR] P-POSSUM mortality risk in ICU-admitted patients (9.4% [6.2–20.1] vs 3.9% [2.5–8.1]; P < 0.001) and more emergency surgery (25 [59.5%] vs 75 [42.6%]; P = 0.048) (ESM, eTable 3); findings with frailty across domains remained, although analyses were constrained by small numbers in the ICU-admitted cohort (ESM, eTables 4 and 5).
Discussion
In a single-centre prospective cohort study, we found strong correlation and agreement between the CFS and the Edmonton Frail Scale in a surgical population. Furthermore, the CFS showed a strong ability to discriminate between people identified as being frail or not frail based on the Edmonton scale. This study also shows that frailty in surgical patients measured by the CFS affects the full spectrum of health, including functional dependence, medication use, physical ability, and nutrition. Consistent with the larger perioperative literature, surgical patients with frailty also had considerably worse postoperative outcomes, with increased complications, higher mortality, and reduced discharge home after surgery.
It is ten years since the NCEPOD recommendation to include frailty screening in surgical risk assessment for older patients.10 Nevertheless, there remains a major gap in perioperative frailty screening. The Association of Anaesthetists of Great Britain and Ireland, the American College of Surgeons, and the American Geriatrics Society all similarly emphasize the importance of perioperative frailty measurement.25,26 The Edmonton Frail Scale has been applied in a variety of surgical cohorts, where it has been found predictive of postoperative complications, increased length of stay, and inability to be discharged home after surgery.27 It also has good inter-rater reliability, and correlates well with a comprehensive geriatric assessment.11 There are significant challenges, however, in its widespread application as a screening tool to a surgical population. Half of our cohort, for example, could not undergo the timed-up-and-go test, with a further 13% unable to undergo clock-drawing cognitive assessment because of their decreased conscious state. There is also a time and training requirement, which presents challenges in a busy preoperative assessment clinic, as well as the emergency surgical setting. Because of these challenges, the Edmonton scale has limited scope for widespread deployment in an unselected population at risk for frailty. The CFS thus presents a convenient, easy to deploy screening tool devoid of many of these constraints. The CFS has been well studied in the perioperative literature, and has been shown to be feasible. In the study by McIsaac et al., it was rated by anesthesiologists as easy to use, useful, and beneficial to patient care, and taking a mean (SD) of 44 (40) sec to administer,14 and was associated with poor outcomes in surgical patients. The AUCROC of 0.91 and high degree of correlation between the CFS and Edmonton scales found in this study supports its role as a screening tool in surgical patients. This study also implies that the threshold for frailty measured by both the CFS and Edmonton scales is similar. Nine of ten divergent patients (Edmonton frail, CFS non-frail) had a CFS score of 4 (“vulnerable”), only one point less than the frailty cut-point of 5. Similarly, 18 of 19 patients scored frail via the CFS and non-frail with the Edmonton scale—their Edmonton score was 6–7 (“vulnerable”), which was just one point less than the frailty cut-point score of 8. This indicates that the CFS adequately selects patients for further assessment with a more detailed, granular multidimensional frailty measure such as the Edmonton scale. Although the highest Youden index was seen for a CFS ≥ “mildly frail” (CFS = 5), we advocate that this screening cut-point should be a CFS ≥ 4, which we would have captured in our cohort in all but one Edmonton-scored patient with frailty. This also represents an appropriate balance between specificity and sensitivity; patients selected at this point will likely have Edmonton scores achieving or very near the “vulnerable” threshold, and will likely also benefit from formalized frailty measurement. This formalized assessment is obviously less applicable to emergency surgical patients. In this population, the finding of significantly increased mortality and postoperative complications with frailty (as with other studies) may instead prompt more attention to a shared decision-making process for frail patients.
A major finding of our study was that perioperative frailty affects a wide range of health domains, with screening able to identify particular areas in which health deficits are over-represented in frail surgical patients. This included functional dependence, medication use, physical performance, and malnutrition, and concurs with our recent study into the areas of health affected by frailty in critical illness.15 This is a significant finding, as it implies that follow-up frailty measurement following a screening process must be comprehensive, particularly given recent trends to use hospital databases to construct so-called “frailty” measures. Although these rapid scales are easy to derive, there may be a significant bias towards medical comorbidities, and comparatively little information regarding other equally important domains of frailty. For example, the “modified frailty index”, an automated frailty measure derived from coding data, which has seen numerous publications in the surgical literature, is almost entirely a comorbidity measure.28 It may therefore fail to capture the complex multidimensional state that is frailty, and risk over-simplifying this complex condition to the detriment of future research and potential development of interventions for frail surgical patients.29,30
More recent examples of better-designed electronic frailty indices exist. The “eFI”, derived and validated in primary-care setting data sets in the UK, is a 36-item frailty index that captures a wide range of health deficits.31 It is able to predict mortality, hospitalization, and nursing home admission, but has not been tested in the perioperative setting. A more recent surgical-specific index, the “perioperative frailty index” has been developed by McIsaac’s group using population-based health administrative data in Canada.32 This index comprises 30-items, and in over half-a-million surgical patients was shown to correlate with postoperative death and institutional discharge. In this new era of electronic health records, electronic frailty indices thus represent an extremely useful approach to measuring frailty, however, must be comprehensive across the spectrum of health.
The finding of specific health domains that are disproportionately affected by frailty may also help identify areas that are potentially modifiable perioperatively. For example, the emerging interest in preoperative physical training (“prehabilitation”) may potentially be able to improve physical performance prior to surgery in frail patients. Limited research has shown this potential, with ongoing studies in this area.33,34 Preoperative nutritional support may also be able to reduce the malnutrition seen in surgical patients with frailty.35,36 More research is needed to determine whether these or other interventions can translate to better postoperative outcomes with frailty. Although logistically challenging for emergency surgery, elective surgical timelines may allow such targeted preoperative optimisation. Moreover, in the postoperative period there may be benefit in addressing specific areas of health deficits for individual patients, to reduce overall operative risk.
Strengths of our study are the inclusion of a broad range of both emergency and elective surgical patients across the spectrum of surgical subspecialties, enhancing generalizability. Although other studies have examined the CFS in specific surgical cohorts, including cardiac surgical37 and general surgical patients,38 the inclusion of a wide range of surgical specialties is previously limited within the literature (an exception being McIsaac et al.’s study of 702 non-cardiac surgical patients).14 A further strength is the similarity between included and non-included patients, indicating that our study cohort is likely representative of surgical patients in our institution, as well as the completeness of study data.
A study limitation was the use of both the Edmonton and Reported Edmonton scales, with total scores possibly varying by one point. Although possibly influencing the comparability of scores obtained, we considered this preferable to missing physical performance scores for half of the cohort (107 of 218 patients), which would have made assessment of this frailty domain impossible. A further limitation was the fact our study was conducted in a single hospital, which may reduce generalizability, and also that investigators assigned both frailty scores without blinding nor randomisation of assessment order, potentially biasing measurement. Nevertheless, we consider the risk of bias to be low since CFS and Edmonton scores were assigned based on objective criteria and strict definitions regarding function, independence, and medical disease status, and total Edmonton scores were aggregated after the CFS score was assigned. Moreover, the methodology in this study (data collectors non-blinded to the comparator scale) is similar to that of other studies comparing frailty scales, including in critically ill and other patient populations.39,40 Future research could improve on our and others’ study designs by utilising blinded assessment in frailty measurement, and by having individual patient frailty measured by more than one investigator to assess inter-rater reliability and to explore potential differences in assessments according to healthcare provider background. For example, a recent study in an ICU population revealed similar CFS scores when assigned by raters from medical, research coordinator, or occupational therapy backgrounds.41 This remains unexplored in the perioperative setting. An extension of the study design to compare the CFS with a comprehensive geriatric assessment in surgical patients would also be of value, although we note the significant challenges in administering such a comprehensive tool in the perioperative setting. Forty-two (19%) of our patients were also included in our previous study examining the Edmonton and CFS in ICU patients. Sensitivity analyses, however, showed little impact of inclusion of these patients on overall findings. In particular, the screening performance of the CFS with respect to the Edmonton scale was maintained, association with frailty and baseline demographics preserved, and the finding of worse health status across frailty domains persisted regardless of inclusion or omission of these patients. Therefore, our conclusions about frailty in the perioperative context remain valid.
In conclusion, the CFS is an accurate, sensitive screening tool, with good face and content validity to measure frailty in the perioperative setting. Frailty in surgical patients affects the spectrum of health-related domains, which are important to include in candidate frailty measurement instruments. Higher risk patients should be screened for frailty prior to anesthesia with a cut-point of a CFS ≥ 4 selecting those for more comprehensive measurement. This may help identify particular health domains amenable to interventions to reduce the impact of perioperative frailty.
References
United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2017. Available from URL: https://www.un.org/en/development/desa/population/theme/ageing/WPA2017.asp (accessed December 2019).
Australian Government; Australian Institute of Health and Welfare. Admitted patient care 2015–16: Australian hospital statistics, Health services series no.75. Cat. no. HSE 185. Canberra: Australian Institute of Health and Welfare; 2017. Available from URL: https://www.aihw.gov.au/getmedia/3e1d7d7e-26d9-44fb-8549-aa30ccff100a/20742.pdf.aspx?inline=true (accessed December 2019).
Yang R, Wolfson M, Lewis MC. Unique aspects of the elderly surgical population: an anesthesiologist’s perspective. Geriatr Orthop Surg Rehabil 2011; 2: 56-64.
Griffiths R, Mehta M. Frailty and anaesthesia: what we need to know. BJA Education 2014; 14: 273-7.
Partridge JS, Harari D, Dhesi JK. Frailty in the older surgical patient: a review. Age Ageing 2012; 41: 142-7.
Oakland K, Nadler R, Cresswell L, Jackson D, Coughlin PA. Systematic review and meta-analysis of the association between frailty and outcome in surgical patients. Ann R Coll Surg Engl 2016; 98: 80-5.
Watt J, Tricco AC, Talbot-Hamon C, et al. Identifying older adults at risk of harm following elective surgery: a systematic review and meta-analysis. BMC Med 2018; DOI: https://doi.org/10.1186/s12916-017-0986-2.
Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146-56.
Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489-95.
National Confidential Enquiry into Patient Outcome and Death. Elective & Emergency Surgery in the Elderly: An Age Old Problem. London: NCEPOD; 2010. Available from URL: https://www.ncepod.org.uk/2010eese.html (accessed December 2019).
Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35: 526-9.
Cooper Z, Rogers SO Jr, Ngo L, et al. Comparison of frailty measures as predictors of outcomes after orthopedic surgery. J Am Geriatr Soc 2016; 64: 2464-71.
Bagshaw SM, Stelfox HT, McDermid RC, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ 2014; 186: E95-102.
McIsaac DI, Taljaard M, Bryson GL, et al. Frailty as a predictor of death or new disability after surgery: a prospective cohort study. Ann Surg 2018; DOI: https://doi.org/10.1097/sla.0000000000002967.
Darvall JN, Greentree K, Braat MS, Story DA, Lim WK. Contributors to frailty in critical illness: multi-dimensional analysis of the Clinical Frailty Scale. J Crit Care 2019; 52: 193-9.
Darvall JN, Braat S, Story DA, et al. Protocol for a prospective observational study to develop a frailty index for use in perioperative and critical care. BMJ Open 2019; DOI: https://doi.org/10.1136/bmjopen-2018-024682.
Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 1983; 31: 721-7.
Prytherch DR, Whiteley MS, Higgins B, Weaver PC, Prout WG, Powell SJ. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enUmeration of mortality and morbidity. Br J Surg 1998; 85: 1217-20.
Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62: 722-7.
Hilmer SN, Perera V, Mitchell S, et al. The assessment of frailty in older people in acute care. Australas J Ageing 2009; 28: 182-8.
Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80: 27-38.
Hartling L, Hamm M, Milne A, et al. Validity and Inter-Rater Reliability Testing of Quality Assessment Instruments 2012. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Mar. Table 2. Interpretation of Fleiss’ kappa (κ) (from Landis and Koch 1977). Available from URL: https://www.ncbi.nlm.nih.gov/books/NBK92295/table/methods.t2/ (accessed December 2019).
Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied Logistic Regression - Third Edition. Wiley; 2013.
Looney SW. Practical issues in sample size determination for correlation coefficient inference. SM J Biometrics Biostat 2018; 3: 1027.
Chow WB, Rosenthal RA, Merkow RP, Ko CY, Esnaola NF; American College of Surgeons National Surgical Quality Improvement Program; American Geriatrics Society. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg 2012; 215: 453-66.
Griffiths R, Beech F, Brown A, et al. Peri-operative care of the elderly 2014: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2014; 69(Suppl 1): 81-98.
Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr 2009; 48: 78-83.
Tsiouris A, Hammoud ZT, Velanovich V, Hodari A, Borgi J, Rubinfeld I. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res 2013; 183: 40-6.
Rodriguez-Manas L, Feart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci 2013; 68: 62-7.
Darvall JN, Pilcher D, Bellomo R, et al. Discussion about “Association of frailty with short-term outcomes, organ support and resource use in critically ill patients”. Intensive Care Med 2018; 44: 2014-6.
Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016; 45: 353-60.
McIsaac DI, Wong CA, Huang A, Moloo H, van Walraven C. Derivation and validation of a generalizable preoperative frailty index using population-based health administrative data. Ann Surg 2019; 270: 102-8.
Milder DA, Pillinger NL, Kam PC. The role of prehabilitation in frail surgical patients: a systematic review. Acta Anaesthesiol Scand 2018; 62: 1356-66.
McIsaac DI, Saunders C, Hladkowicz E, et al. PREHAB study: a protocol for a prospective randomised clinical trial of exercise therapy for people living with frailty having cancer surgery. BMJ Open 2018; DOI: https://doi.org/10.1136/bmjopen-2018-022057.
Barberan-Garcia A, Ubre M, Roca J, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018; 267: 50-6.
Gillis C, Wischmeyer PE. Pre-operative nutrition and the elective surgical patient: why, how and what? Anaesthesia 2019; 74(Suppl 1): 27-35.
Lytwyn J, Stammers AN, Kehler DS, et al. The impact of frailty on functional survival in patients 1 year after cardiac surgery. J Thorac Cardiovasc Surg 2017; 154: 1990-9.
Hewitt J, Moug SJ, Middleton M, Chakrabarti M, Stechman MJ, McCarthy K; Older Persons Surgical Outcomes Collaboration. Prevalence of frailty and its association with mortality in general surgery. Am J Surg 2015; 209: 254-9.
Bagshaw S, Ibrahim Q, Majumdar S, et al. Association between frailty and short and long-term outcomes in critically ill patients. Crit Care Med 2013; DOI: https://doi.org/10.1097/01.ccm.0000439682.51432.90.
Le Maguet P, Roquilly A, Lasocki S, et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med 2014; 40: 674-82.
Shears M, Takaoka A, Rochwerg B, et al. Assessing frailty in the intensive care unit: a reliability and validity study. J Crit Care 2018; 45: 197-203.
Author contributions
Jai N. Darvall contributed to the study design, patient recruitment, analysis of data, and manuscript drafting. Joel Loth and Tony Bose contributed to study design, patient recruitment, and manuscript review. Sabine Braat and Anurika De Silva contributed to data analysis and manuscript review. David A. Story and Wen K. Lim contributed to the study design and manuscript review.
Conflicts of interest
None.
Funding statement
This work was supported by an emerging researcher scholarship from the Australian and New Zealand College of Anaesthetists.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Darvall, J.N., Loth, J., Bose, T. et al. Accuracy of the Clinical Frailty Scale for perioperative frailty screening: a prospective observational study. Can J Anesth/J Can Anesth 67, 694–705 (2020). https://doi.org/10.1007/s12630-020-01610-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-020-01610-x