Abstract
Purpose
The high-thoracic erector spinae plane block (HT-ESPB) has been reported as an effective analgesic modality for the shoulder region without phrenic nerve palsy. The goal of this study was to compare the HT-ESPB as a phrenic nerve-sparing alternative to an interscalene block for total shoulder arthroplasty.
Methods
Thirty patients undergoing total shoulder arthroplasty at Stanford Health Care (Palo Alto, CA, USA) were enrolled in a double-blind randomized controlled trial. We randomized 28 patients to receive either an interscalene or HT-ESPB perineural catheter preoperatively; 26 patients were included in the final analysis. The study was powered for the primary outcome of incidence of hemidiaphragmatic paralysis in the postanesthesia care unit (PACU). Other outcome measures included incentive spirometry volume, brachial plexus motor and sensory exams, adverse events, pain scores, and opioid consumption.
Results
The incidence of hemidiaphragmatic paralysis in the HT-ESPB catheter group was significantly lower than in the interscalene catheter group (0/12, 0% vs 14/14, 100%; P < 0.001). No statistically significant differences were found in pain scores and opioid consumption (in oral morphine equivalents) between the interscalene and HT-ESPB groups through postoperative day (POD) 2. Nevertheless, the mean (standard deviation) point estimates for opioid consumption for the HT-ESPB group were higher than for the interscalene group in the PACU (HT-ESPB: 24.8 [26.7] mg; interscalene: 10.7 [21.7] mg) and for POD 0 (HT-ESPB: 20.5 [25.0] mg; interscalene: 6.7 [12.0] mg). In addition, cumulative postoperative opioid consumption was significantly higher at POD 0 (PACU through POD 0) in the HT-ESPB group (45.3 [39.9] mg) than in the interscalene group (16.6 [21.9] mg; P = 0.04).
Conclusions
This study suggests that continuous HT-ESPB can be a phrenic nerve-sparing alternative to continuous interscalene brachial plexus blockade, although the latter provided superior opioid-sparing in the immediate postoperative period. This was a small sample size study, and further investigations powered to detect differences in analgesic and quality of recovery score endpoints are needed.
Study registration
www.ClinicalTrials.gov (NCT03807505); registered 17 January 2019.
Résumé
Objectif
Le bloc des muscles érecteurs du rachis du haut thorax (BMER-HT) a été rapporté comme une modalité analgésique efficace pour la région de l’épaule et ce, sans paralysie du nerf phrénique. L’objectif de cette étude était de comparer ce bloc en tant qu’alternative épargnant le nerf phrénique à un bloc interscalénique pour l’arthroplastie totale de l’épaule.
Méthode
Trente patients bénéficiant d’une arthroplastie totale de l’épaule au centre de soins Stanford Health Care (Palo Alto, CA, États-Unis) ont été recrutés dans une étude randomisée contrôlée à double insu. Nous avons randomisé 28 patients à recevoir un cathéter périneural interscalénique ou un BMER-HT en préopératoire; 26 patients ont été inclus dans l’analyse finale. Le calcul de puissance de l’étude a été effectué pour répondre au critère d’évaluation principal, qui était l’incidence de paralysie hémidiaphragmatique en salle de réveil. Les autres issues mesurées comprenaient les volumes de spirométrie, les examens moteurs et sensoriels du plexus brachial, les événements indésirables, les scores de douleur et la consommation d’opioïdes.
Résultats
L’incidence de paralysie hémidiaphragmatique dans le groupe cathéter BMER-HT était significativement plus faible que dans le groupe cathéter interscalénique (0/12, 0 % vs 14/14, 100 %; P < 0,001). Aucune différence statistiquement significative n’a été observée dans les scores de douleur et la consommation d’opioïdes (en équivalents morphine par voie orale) entre les groupes interscalénique et BMER-HT jusqu’au jour postopératoire (JPO) 2. Néanmoins, en salle de réveil, les estimations ponctuelles moyennes (écart type) de la consommation d’opioïdes pour le groupe BMER-HT étaient plus élevées que pour le groupe interscalénique (BMER-HT : 24,8 [26,7] mg; interscalénique : 10,7 [21,7] mg), ainsi qu’au JPO 0 (BMER-HT : 20,5 [25,0] mg; interscalénique: 6,7 [12,0] mg). De plus, la consommation cumulative d’opioïdes postopératoires était significativement plus élevée au JPO 0 (salle de réveil jusqu’au JPO 0) dans le groupe BMER-HT (45,3 [39,9] mg) que dans le groupe interscalénique (16,6 [21,9] mg; P = 0,04).
Conclusion
Cette étude suggère que le BMER-HT continu peut être une alternative au bloc interscalénique continu du plexus brachial pour épargner le nerf phrénique, bien que le bloc interscalénique ait fourni une épargne d’opioïdes supérieure en période postopératoire immédiate. Il s’agissait d’une étude de petite taille d’échantillon, et d’autres études visant à détecter les différences dans les scores des critères d’évaluation en matière d’analgésie et de qualité de la récupération sont nécessaires.
Enregistrement de l’étude
www.clinicaltrials.gov (NCT03807505); enregistrée le 17 janvier 2019.
Similar content being viewed by others
Major shoulder surgery can cause severe pain, and the interscalene brachial plexus nerve block is commonly used to provide analgesia.1 Continuous interscalene blockade (CISB) provides superior pain relief over parenteral opioid analgesia2 and reduces the time to discharge.3 Nevertheless, ipsilateral phrenic nerve palsy occurs in up to 100% of patients. Even with contemporary ultrasound-guided techniques and lower local anesthetic volumes, 100% partial or full hemidiaphragm paresis has been seen on ultrasound evaluation.4,5,6 This can lead to shortness of breath at rates between 9 and 12% in study patients.7,8 Concern for respiratory compromise in patients with pulmonary disease, morbid obesity, or phrenic nerve dysfunction can be a reason to forego an interscalene block. Investigations into phrenic nerve-sparing blocks have included reducing local anesthetic volume and targeting more distal points along the brachial plexus.9,10 Nevertheless, a completely diaphragm-sparing block has not been identified.
The erector spinae plane (ESP) block has predominantly been described for analgesia at the thoracic and lumbar levels.11 More recently, the use of the high-thoracic ESP block (HT-ESPB) for postoperative analgesia of the shoulder and upper extremity has been described in several case reports, including for forequarter amputation,12 total shoulder arthroplasty (TSA), and proximal humerus surgery.13 Radiographic spread of injectate at the mid/high-thoracic level of the ESP has been shown to reach the cervical neural foramina and nerve root area.14,15 The HT-ESPB has been suggested as a phrenic nerve-sparing alternative to the interscalene block. A cadaver study assessing the cervical (C6 and C7) ESP block16 showed deep staining of the phrenic nerve in 10% of injections and faint staining in 20%. Local anesthetic from the HT-ESPB is introduced at a more caudal (T1/2) level, which infers an even lower risk of phrenic nerve involvement.
In this study, we sought to evaluate whether a HT-ESPB catheter is a phrenic nerve-sparing analgesic alternative to the interscalene brachial plexus catheter for TSA. We hypothesized that patients receiving the HT-ESPB catheter would have a lower incidence of hemidiaphragm paresis compared with patients receiving an interscalene catheter.
Methods
We conducted a double-blinded randomized controlled trial at Stanford Health Care (Palo Alto, CA, USA). The study was registered with ClinicalTrials.gov (NCT03807505) on 17 January 2019. After Institutional Review Board (Stanford Health Care) approval in May 2019, a total of 30 adult patients undergoing elective primary TSA were enrolled. Exclusion criteria included significant pulmonary disease, coagulopathy, contraindication to nerve block, chronic opioid use, body mass index > 40 kg·m-2, and prior cervical or thoracic spine surgery. Patients were contacted at least one day prior to surgery to discuss the study rationale, and written informed consent was obtained in the preoperative area on the day of surgery by the research team. Patients were assigned to groups using a computer-generated 1:1 ratio randomization schedule with sealed envelopes given to the regional anesthesia team by study investigators. The study was powered for the primary outcome of incidence of hemidiaphragmatic paralysis assessed by ultrasound in the postanesthesia care unit (PACU).
Blinding
Research assistants (who performed recruitment and all data collection) and patients were blinded to randomization. Patients agreed to be blinded to whether they received the “usual” catheter (interscalene; control) or the “other” catheter (ESP) and had no pre-existing knowledge of the difference in block sites. The possible interscalene block site (usually visible during data collection) was covered with gauze by an unblinded study investigator before post-block data collection. All direct patient care providers maintained patient blinding and were aware of patient allocation to ensure that appropriate care was provided.
Peripheral nerve catheter placement
All nerve catheters were placed under sterile conditions in the preoperative holding area with standard American Society of Anesthesiologists monitors, supplemental oxygen, and intravenous sedation (fentanyl and midazolam) titrated to a Ramsay Sedation Scale score of 2 or 3. A perineural bolus of 10 mL of 0.5% ropivacaine was administered preoperatively, immediately after catheter placement.
The interscalene catheter was placed with patients in a semirecumbent position. After identification of the cervical roots (C5 and C6) using a high-frequency linear ultrasound transducer (LOGIQ™ S8, GE Healthcare, Chicago, IL, USA), a 17G Tuohy needle was inserted, lateral to medial, with a short axis in-plane technique and placed adjacent to the nerves. A 19G wire-reinforced catheter (FlexBlock™, Arrow, Teleflex Incorporated, Morrisville, NC, USA) was advanced 1–2 cm past the tip of the Tuohy under ultrasound visualization. Isotonic sodium chloride (1–5 mL) was injected through the catheter to confirm spread around the C5 and C6 roots.
The HT-ESPB catheter was placed with patients in a prone position. After identification of the T4 or T5 transverse process, whichever was best visualized, using a high-frequency linear ultrasound transducer (LOGIQ S8), a 17G Tuohy needle was inserted, caudal to cranial, with an in-plane approach and advanced to contact the transverse process (Figure). The needle was then slightly withdrawn, and isotonic sodium chloride (25–30 mL) was injected for hydrodissection of the erector spinae fascial plane. This resulted in injectate spread cephalad and caudad, lifting up the erector spinae muscles and facilitating catheter advancement. After estimating the distance from the site of needle puncture to the superior border of the scapula (surface landmark for T1/T2), the catheter (FlexBlock) was threaded smoothly in the cephalad direction typically between 10 and 15 cm past the tip of the Tuohy, until the estimated distance was reached. If resistance was met prior to the estimated distance, the catheter was removed and further hydrodissection of the ESP under ultrasound confirmation was performed prior to replacement of the catheter. To confirm catheter location, isotonic sodium chloride (1–5 mL) was injected through the catheter under ultrasound visualization to verify spread in the ESP at the T1/T2 level or higher.
Ultrasound image of a high-thoracic erector spinae plane catheter placement using a Tuohy needle. A 17G Tuohy needle (white arrow) was inserted, caudal to cephalad, with an in-plane approach and advanced until the needle tip (black arrow) made contact with the transverse process (TP). The needle was then slightly withdrawn, and 25–30 mL of saline was utilized for hydrodissection of the erector spinae fascial plane. This resulted in injectate spreading (***) cephalad and caudad, lifting up the erector spinae muscles (ESM)
Intraoperative and postoperative management
All patients received a general anesthetic with endotracheal intubation for the surgical procedure by an anesthesiologist independent from the study. Intraoperative anesthesiologists gave an additional 10 mL ropivacaine 0.5% to administer as a one-time bolus in the nerve catheter prior to giving opioid if there was tachycardia or hypertension thought to be related to surgical stimulus. Intraoperative opioid use consisted of fentanyl prior to intubation, and additional intravenous fentanyl or hydromorphone given for tachycardia or hypertension from surgical stimulus.
On arrival at the PACU, 5 mL of 0.5% ropivacaine was administered and PACU measurements were taken 30 min later. Patients with interscalene catheters received 0.2% ropivacaine by an automated bolus of 6 mL every hour. Patients with HT-ESPB catheters received an automated bolus of 0.2% ropivacaine at 12 mL every two hours. Both groups had a patient demand bolus of 5 mL with a lockout time of 30 min.
All patients were followed by our institution’s acute pain service postoperatively. Standardized postoperative multimodal analgesia included acetaminophen, gabapentin, oxycodone, and hydromorphone.
Perineural catheters were continued through at least postoperative day (POD) 2. Patients remained blinded for final data collection on POD 3. Patients were discharged after meeting discharge criteria.
Pulmonary function
The outcome of hemidiaphragm paralysis was assessed using the diaphragmatic thickening ratio. This is a measurement of the relative increase in diaphragm thickness with deep inspiration compared with expiration and was calculated as the difference between hemidiaphragm thickness at deep inspiration and expiration, divided by hemidiaphragm thickness at expiration. Hemidiaphragm thickness was measured by ultrasound using the ABCDE technique17 by research assistants. Measurements were performed pre-block and in the PACU.
Hemidiaphragmatic paralysis was categorized as none, partial, or full. “None” referred to 0 to 25% change in the diaphragmatic thickening ratio from pre-block to PACU. “Partial” referred to 25% to 75% decrease in the diaphragmatic thickening ratio from pre-block to PACU. “Full” referred to a more than 75% decrease in the diaphragmatic thickening ratio from pre-block to PACU.9
Incentive spirometry volumes were measured at three time points: pre-block, PACU, and POD 1. Three measurements were taken each time, and the average of these volumes was calculated for each time point.
Numerical rating scale pain and opioid consumption
Pain scores (numerical rating scale [NRS], 0–10) were assessed per institutional routine by bedside nurses every four hours and with pain medication requests. As pain scores were not normally distributed, the median pain scores during the time intervals of PACU, POD 0, POD 1, and POD 2 were calculated for each patient. The average of these median pain scores for each time interval was compared between the two groups. Opioid consumption (oral morphine equivalents [OME]) was collected by chart review. For cumulative opioid consumption calculations, arrival to the PACU was used as the starting time point.
Motor and sensory function
Brachial plexus motor function was assessed with finger extension, finger abduction, and thumb opposition to resistance. Decrease in motor function was categorized as minimal, moderate, or full in the PACU (minimal decrease: able to move against some resistance in all areas, moderate decrease: able to move against gravity in some areas, full decrease: no contraction in all areas). Brachial plexus sensory function was assessed by temperature sensation to cold spray in the C5–C8 dermatomes. Decrease in sensory function was categorized as minimal, moderate, or full in the PACU (minimal decrease: some cold sensation throughout, moderate decrease: absence of cold sensation in any dermatome, full decrease: absence of cold sensation in all dermatomes). The Activity Measure for Post-Acute Care “6-Clicks” daily activity domain score to assess patient mobility and activity limitations18 was documented by an occupational therapist on POD 1.
Adverse effects and patient satisfaction
The incidence of dyspnea, Horner’s syndrome, hoarseness, patient-reported difficulty participating in physical therapy due to motor and/or sensory block, and satisfaction with the nerve block was collected using a standardized questionnaire during the POD 1 visit and during the POD 3 interview (in person or by phone if discharged). The incidence of postoperative nausea, as a measure of opioid-related adverse effects, was assessed during post hoc analysis by collecting antiemetic medication administration (from PACU to POD 2) during chart review.
Statistical analysis
The interscalene block can cause a 100% incidence of phrenic nerve palsy.4,5,6 A clinically meaningful reduction in the incidence of phrenic nerve palsy (“partial” or “full” hemidiaphragmatic paralysis by ultrasound measurement) was determined to be a minimum difference of 50%. Eleven patients per group were required for 80% power at an alpha level of 0.05. To compensate for attrition, 15 patients per group were targeted for enrollment. Categorical outcomes were compared using Fisher’s exact test. For continuous outcomes, a Shapiro test was performed to check for normality. If both variables were normally distributed, a t test was performed to obtain the P value. If data were not normally distributed, a Kolmogorov–Smirnov test was performed to evaluate whether the two variables had similar distributions. If the distributions were similar, then a Wilcox test was performed to obtain the P value. Results with P < 0.05 were considered statistically significant. Data were analyzed using RStudio (Boston, MA, USA).
Results
Participant flow
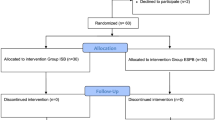
After informed consent, 30 patients were enrolled, with 15 patients per group (Figure). Recruitment and follow-up occurred from June to November 2019. Prior to randomization, one patient was excluded after delayed disclosure of chronic opioid use. One patient was excluded because of undiagnosed cardiopulmonary disease (received a superior trunk catheter and required supplemental oxygen for hypoxia and dyspnea in PACU). After randomization, one HT-ESPB group patient was removed because of unblinding before block placement. One HT-ESPB group patient received an interscalene catheter because the catheter could not be threaded sufficiently cephalad to achieve saline spread at the T1/2 level after three attempts. In total, 12 patients in the HT-ESPB group and 14 patients in the interscalene group completed the study per protocol. There was no significant difference in baseline characteristics between the two groups (Table 1).
There were some notable participant events. One patient in the HT-ESPB group had a catheter inadvertently threaded 15 cm past the Tuohy and had poorly controlled pain in PACU, so the catheter was pulled back 5 cm and bolused with 10 mL ropivacaine 0.5% with satisfactory analgesia. One interscalene group patient had shortness of breath and difficulty clearing secretions, so the catheter infusion was changed to a continuous rate of 5 mL·hr-1 of ropivacaine 0.2%. One patient in the interscalene group had ptosis ipsilateral to the nerve block and underwent a stroke-protocol head computed tomography, which was unremarkable. The catheter infusion was paused, with resolution of the ptosis, and restarted.
Hemidiaphragmatic paralysis
There was a lower incidence of partial or full hemidiaphragmatic paralysis in the HT-ESPB group compared with the interscalene group (0/12, 0% vs 14/14, 100%; P < 0.001) (Table 2).
Incentive spirometry volume
There was a larger percent decrease in incentive spirometry volume in the interscalene group compared with the HT-ESPB group from baseline to PACU (44% vs 9%; P < 0.001) and from baseline to POD 1 (38% vs 7%; P < 0.001) (Table 2).
Pain scores and opioid consumption
There was no significant difference in the observed pain scores and opioid consumption (Table 3) in the PACU or on POD 0, POD 1, or POD 2. Nevertheless, there was a trend towards higher mean (standard deviation [SD]) postoperative opioid consumption (OME) in the discrete PACU and POD 0 periods in the HT-ESPB group compared with the interscalene group (PACU: 24.8 [26.7] mg vs 10.7 [21.7] mg; POD 0: 20.5 [25.0] mg vs 6.7 [12.0] mg), as well as a trend towards higher mean (SD) pain scores in those periods (PACU: 3.8 [3.1] vs 1.3 [2.0]; POD 0: 2.4 [1.7] vs 1.4 [2.3]). In addition, the mean (SD) cumulative postoperative opioid consumption was significantly higher at POD 0 (PACU through POD 0) in the HT-ESPB group than in the interscalene group (16.6 [21.9] mg vs 45.3 [39.9] mg; P = 0.04). There was no significant difference in cumulative opioid consumption at POD 2 (PACU through POD 2) between the two groups (54.4 [64.3] mg vs 93.6 [84.9] mg; P = 0.08).
Adverse events
There was no difference in discrete patients with any adverse event (block-related events and/or postoperative antiemetic use) (Table 4). The interscalene group had more patients with any block-related events (4/14 vs 0/12, P = 0.03).
Discussion
This randomized trial found that all patients who received a HT-ESPB catheter preserved their baseline respiratory function. Patients who received an interscalene catheter all had partial or full hemidiaphragm paralysis and a greater decline in incentive spirometry volumes. There was no significant difference in pain scores at POD 2. Patients in the HT-ESPB group had higher cumulative opioid consumption at POD 0. This suggests that the HT-ESPB can be a phrenic-sparing alternative to the interscalene brachial plexus block, although there is superior opioid-sparing effect from an interscalene catheter in the immediate postoperative period.
While fascial plane blocks can achieve certain level of analgesic effect, most experts believe that this effect is less dense compared with that of peripheral nerve, plexus, or central neuraxial blockade of both visceral and somatic pain.19 Hence, as one might anticipate, the findings of this study also showed that there was a superior analgesic effect with a brachial plexus block from an interscalene catheter compared with a fascial plane block from an ESP catheter. Nevertheless, it is important to note that this study has a small sample size and was intended to be a preliminary investigation of the potential for the HT-ESPB to be a phrenic nerve-sparing block and was powered to detect a difference in hemidiaphragm paralysis.
Based on a prior cadaver study,16 the spread of injectate from a high-thoracic catheter could potentially involve the phrenic nerve, which was the basis for this study. The lack of phrenic nerve involvement found in this study can be explained by a combination of the lower level of the catheter as well as the difference in tissue spread between a cadaver and a live human model. In addition, the 10% rate of phrenic nerve staining observed in the cadaveric study was from direct injection through a needle, which typically has a wider spread than injection through a catheter, as in this study.20
This study has a number of limitations. It was not powered to detect a difference in opioid consumption or pain scores, and a future study powered for these important outcomes would better measure and describe any difference in analgesic effect as well as the quality of recovery scores between the two techniques. While no statistical difference was reached in either pain scores or opioid consumption in the PACU and at POD 1, it is important to point out that the trend towards higher pain scores and opioid consumption during the immediate postoperative period indicates that optimization of multimodal analgesia is needed when utilizing the HT-ESPB technique. Nevertheless, our study was not powered to detect a difference in these measures as noted. The healthcare providers of the patients were not blinded because we did not want to compromise patient care when managing a newer regional anesthetic technique. We did not employ a correction for multiple comparisons. Given the limits of the smaller sample size in this study, we did not want to further increase the susceptibility to type II errors.
Another limitation of this study was the absence of a placebo control group due to ethical considerations because the standard practice at our institution is to provide an interscalene catheter for TSA, which hence served as our control. Given this limitation, we performed a comparison analysis through a literature review of studies with patient cohorts that received no regional anesthesia. Ilfeld et al. randomized patients undergoing TSA to interscalene catheters containing saline or ropivacaine.3 The placebo group had a significantly higher average NRS pain score on POD 1 (4 vs 1). Opioid consumption for the placebo group was 74 mg OME for the POD 0–1 period (excluding PACU), and 50 mg OME for the POD 1–2 period (extrapolated from Figure 4E and F). In a retrospective case-control study by Ilfeld et al., patients who underwent TSA without a block were matched with patients who received a CISB.21 For the first 24 hr after surgery, the CISB group had a median [interquartile range] pain score of 1 [0.0–6.4] at rest and 2 [0–8.7] with movement, compared with 6 [0.3–9.6] at rest and 8.5 [1.8–10] with movement for the non-block group. YaDeau et al. published a prospective observational study of patients who underwent TSA with a single-injection interscalene block of bupivacaine with adjuvants.22 Opioid use peaked in the second 24-hr period postoperatively (presumably after the nerve block had worn off), with a mean (SD) 24-hr opioid consumption of 82 (74.1) mg (OME). As a crude substitute for a placebo control group, the data from these publications compared with similar time points in our study suggest that patients undergoing TSA without regional anesthesia would have higher pain scores and opioid consumption than the average NRS pain scores of 2.2–2.4 and the average 19–29 mg OME daily opioid consumption seen in our HT-ESPB group from POD 0 through POD 2.
In the literature, there is a wide range of perioperative opioid consumption reported in patients undergoing TSA with CISB. The large variability in opioid administered during the perioperative course can be attributed to differences in institutional practice. The interscalene catheter group in our study had an average first 24-hour opioid consumption total of 60.6 mg OME (43.2 mg intraoperatively, 10.7 mg in PACU, and 6.7 mg in POD 0). With regard to intraoperative opioid administration, it is common practice at our institution to administer 100 mcg fentanyl for intubation, which accounts for 30 mg OME. Additional intraoperative opioid administered was at the discretion of the intraoperative anesthesia team, to allow for differences in anesthetic practice styles. If intraoperative opioid administration is excluded, the first 24-hr opioid consumption of 17.4 mg OME in this study was in fact comparable to, or less than, the 31.8 mg OME (10.6 mg iv morphine) reported by Auyong et al.7 for their interscalene catheter group.
In a cadaver study,16 we previously showed that ultrasound-guided cervical (C6 and C7) ESP injections consistently stained the roots of the brachial plexus and the suprascapular nerve. The HT-ESPB leaves local anesthetic at the T1/2 level or higher (verified during catheter placement). This likely provides cephalad and anterior spread to the brachial plexus, as well as to the suprascapular nerve, which courses laterally at the T1 level to provide sensory innervation of the acromioclavicular and glenohumeral joints. Blockade of the suprascapular nerve, to a greater extent than the brachial plexus, may explain why the HT-ESPB achieves an analgesic effect at the shoulder joint without demonstrable cervical dermatomal sensory changes. The analgesic effect of the HT-ESPB mirrors the results of a metanalysis suggesting that the suprascapular nerve block has less respiratory and block-related complications than the interscalene nerve block, with the interscalene block offering a small analgesic advantage in the immediate postoperative PACU period.8
The placement of a HT-ESPB catheter can be challenging. One patient in the HT-ESPB group was switched to an interscalene catheter because of difficulty threading the catheter cephalad, possibly due to fascial plane anomalies. In another patient, the catheter threaded easily to 15 cm past the tip, with spread of saline visualized above T1/2. The patient had poor analgesia postoperatively, which resolved after the catheter was pulled back 5 cm. We speculated the catheter was placed in the high cervical region. This may suggest that the ideal location of the HT-ESPB catheter is no higher than the low cervical region. In this study, we found it very challenging to obtain clear ultrasound documentation of the final catheter tip position or the full cephalad extent of injectate spread with saline injected via the catheter (beyond T1/2). Instead, we relied on tactile indications that the catheter was being advanced smoothly in the fascial plane, as suggested by feeling minimum or no resistance with threading the catheter. In addition, the catheter stylet was confirmed to have remained straight without any bending upon removal from the catheter. Any resistance during threading might suggest that the catheter was coiling into muscle instead of advancing through the fascial plane.23
In summary, patients with HT-ESPB catheters had no ultrasonographic evidence of phrenic nerve palsy. Although this study was not powered to detect a difference in opioid consumption, the interscalene catheter did provide an advantage in opioid-sparing over the HT-ESPB catheter during the immediate postoperative period (PACU through POD 0). Overall, the HT-ESPB catheter provided satisfactory analgesia, with reasonable pain scores and opioid consumption beyond POD 1. When comparing historical data from previously published studies with patients undergoing TSA without regional anesthesia, our findings support the HT-ESPB catheter as a viable potential analgesic alternative, particularly in cases when the risk of phrenic nerve palsy is of significant concern. In these clinical scenarios, however, careful optimization of a multimodal analgesic regimen will be needed to improve analgesia, particularly for the immediate postoperative period. It is encouraging that the HT-ESPB did not show phrenic nerve involvement in this preliminary study, although further investigations powered to detect differences in pain, opioid consumption, quality of recovery scores, and other functional outcomes are needed to fully inform the use of this regional anesthetic technique.
References
Gabriel RA, Nagrebetsky A, Kaye AD, Dutton RP, Urman RD. The patterns of utilization of interscalene nerve blocks for total shoulder arthroplasty. Anesth Analg 2016; 123: 758-61.
Ullah H, Samad K, Khan FA. Continuous interscalene brachial plexus block versus parenteral analgesia for postoperative pain relief after major shoulder surgery. Cochrane Database Syst Rev 2014; DOI: https://doi.org/10.1002/14651858.CD007080.pub2.
Ilfeld BM, Vandenborne K, Duncan PW, et al. Ambulatory continuous interscalene nerve blocks decrease the time to discharge readiness after total shoulder arthroplasty: a randomized, triple-masked, placebo-controlled study. Anesthesiology 2006; 105: 999-1007.
Urmey WF, Talts KH, Sharrock NE. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg 1991; 72: 498-503.
Riazi S, Carmichael N, Awad I, Holtby RM, McCartney CJ. Effect of local anaesthetic volume (20 vs 5 ml) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth 2008; 101: P549-56.
Gerber LN, Sun LY, Ma W, et al. Clinical effect of normal saline injectate into interscalene nerve block catheters given within one hour of local anesthetic bolus on analgesia and hemidiaphragmatic paralysis. Reg Anesth Pain Med 2021; 46: 124-9.
Auyong DB, Yuan SC, Choi DS, Pahang JA, Slee AE, Hanson NA. A double-blind randomized comparison of continuous interscalene, supraclavicular, and suprascapular blocks for total shoulder arthroplasty. Reg Anesth Pain Med 2017; 42: 302-9.
Hussain N, Goldar G, Ragina N, Banfield L, Laffey JG, Abdallah FW. Suprascapular and interscalene nerve block for shoulder surgery: a systematic review and meta-analysis. Anesthesiology 2017; 127: 998-1013.
Kim DH, Lin Y, Beathe JC, et al. Superior trunk block: a phrenic-sparing alternative to the interscalene block: a randomized controlled trial. Anesthesiology 2019; 131: 521-33.
Kang RA, Jeong JS, Chin KJ, et al. Superior trunk block provides noninferior analgesia compared with interscalene brachial plexus block in arthroscopic shoulder surgery. Anesthesiology 2019; 131: 1316-26.
Tsui BC, Fonseca A, Munshey F, McFadyen G, Caruso TJ. The erector spinae plane (ESP) block: a pooled review of 242 cases. J Clin Anesth 2019; 53: 29-34.
Tsui BC, Mohler D, Caruso TJ, Horn JL. Cervical erector spinae plane block catheter using a thoracic approach: an alternative to brachial plexus blockade for forequarter amputation. Can J Anesth 2019; 66: 119-20.
Ma W, Sun L, Ngai L, et al. Motor-sparing high-thoracic erector spinae plane block for proximal humerus surgery and total shoulder arthroplasty surgery: clinical evidence for differential peripheral nerve block? Can J Anesth 2019; 66: 1274-5.
Chin KJ, Adhikary S, Sarwani N, Forero M. The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair. Anaesthesia 2017; DOI: https://doi.org/10.1111/anae.13814.
Forero M, Rajarathinam M, Adhikary SD, Chin KJ. Erector spinae plane block for the management of chronic shoulder pain: a case report. Can J Anesth 2018; 65: 288-93.
Elsharkawy H, Ince I, Hamadnalla H, Drake RL, Tsui BC. Cervical erector spinae plane block: a cadaver study. Reg Anesth Pain Med 2020; DOI: https://doi.org/10.1136/rapm-2019-101154.
Khurana J, Gartner SC, Naik L, Tsui BC. Ultrasound identification of diaphragm by novices using ABCDE technique. Reg Anesth Pain Med 2018; DOI: https://doi.org/10.1097/AAP.0000000000000718.
Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-clicks” inpatient daily activity and basic mobility short forms. Phys Ther 2014; 94: 379-91.
Elsharkawy H, Pawa A, Mariano ER. Interfascial plane blocks: back to basics. Reg Anesth Pain Med 2018; DOI: https://doi.org/10.1097/AAP.0000000000000750.
Fujii T, Shibata Y, Ban Y, et al. A single paravertebral injection via a needle vs. a catheter for the spreading to multiple intercostal levels: a randomized controlled trial. J Anesth 2020; 34: 72-8.
Ilfeld BM, Wright TW, Enneking FK, Morey TE. Joint range of motion after total shoulder arthroplasty with and without a continuous interscalene nerve block: a retrospective, case-control study. Reg Anesth Pain Med 2005; 30: 429-33.
YaDeau JT, Dines DM, Liu SS, et al. What pain levels do TSA patients experience when given a long-acting nerve block and multimodal analgesia? Clin Orthop Relat Res 2019; 477: 622-32..
Xu L, Leng JC, Mariano ER, Tsui BC. Erector spinae plane: a collapsible potential space. Reg Anesth Pain Med 2020; 45: 562-3.
Author contributions
Lisa Y. Sun contributed to study design/conception; data acquisition, analysis and interpretation; and manuscript preparation. Shurthi Basireddy contributed to data acquisition, data analysis, and manuscript preparation. Lynn Ngai Gerber contributed to study design, data interpretation, and manuscript preparation. Jason Lamano contributed to data acquisition and data analysis. Emilie Cheung, John Costouros, Jan Boublik, and Jean-Louis Horn contributed to study design/conception and manuscript review. Ban Chi-Ho Tsui contributed to study design/conception, data interpretation, and manuscript preparation.
Disclosures
None.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Deputy Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, L.Y., Basireddy, S., Gerber, L.N. et al. Continuous interscalene versus phrenic nerve-sparing high-thoracic erector spinae plane block for total shoulder arthroplasty: a randomized controlled trial. Can J Anesth/J Can Anesth 69, 614–623 (2022). https://doi.org/10.1007/s12630-022-02216-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02216-1