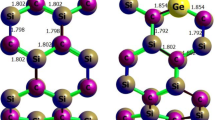
Abstract
The ability of carbon- and silicon-based nanotubes, including pure carbon, silicon carbide, and Ge-doped silicon carbide nanotubes (CNT, SiCNT, SiCGeNT, respectively), for sensing highly toxic dichlorosilane (H2SiCl2) are investigated using quantum chemistry calculations. The intermolecular interactions between the sensing material and the gas molecule have been investigated with the density functional theory calculations with a functional that includes dispersion terms. The selected method employed is B3LYP-D3 (GD3BJ)/6-311G(d), while other functionals including PBE0, ωB97XD, and M06-2X have been used for comparison. The quantum theory of atoms in molecules (QTAIM) analysis is employed to check the type of intermolecular interactions. Natural bond orbital (NBO) calculations have been used to deduce the bond orders. The findings of this work indicate that the adsorption of the H2SiCl2 is a physisorption process, which is very desirable for its function as a sensing element. The Ge-doped nanotube offers maximum adsorption energy in comparison to CNT and SiCNT.
Similar content being viewed by others
Data Availability
Not applicable.
References
Kaushik A, Kumar R, Jayant RD, Nair M (2015) Nanostructured gas sensors for health care: An overview. J Personalized Nanomed 1:10
Dallinger D, Kappe CO (2017) Lab-scale production of anhydrous diazomethane using membrane separation technology. Nat Protoc 12:2138–2147
Ishii A, Yamamoto M, Asano H, Fujiwara K (2008) DFT calculation for adatom adsorption on graphene sheet as a prototype of carbon nanotube functionalization, Journal of Physics: Conference Series, IOP Publishing, pp 052087
DoustMohammadi M, Abdullah HY (2020) The adsorption of chlorofluoromethane on pristine, and Al-and Ga-doped boron nitride nanosheets: a DFT, NBO, and QTAIM study. J Mol Model 26:287
Mohammadi MD, Abdullah HY, Suvitha A (2021) The adsorption of 1-chloro-1, 2, 2, 2-tetrafluoroethane onto the pristine, Al-, and Ga-doped boron nitride nanosheet. Iran J Sci Technol Transac A Sci 45:1287–1300
Hurst J (2014) Boron nitride nanotubes, silicon carbide nanotubes, and carbon nanotubes—a comparison of properties and applications, nanotube superfiber materials. Elsevier, pp 267–287
Vorotyntsev V, Mochalov G, Kolotilova M, Volkova E (2006) Gas-chromatographic and mass-spectrometric determination of impurity hydrocarbons in organochlorine compounds and dichlorosilane. J Anal Chem 61:883–888
Seyferth D, Prud’homme C, Wiseman GH (1983) Cyclic polysiloxanes from the hydrolysis of dichlorosilane. Inorg Chem 22:2163–2167
Walch SP, Dateo CE (2001) Thermal decomposition pathways and rates for silane, chlorosilane, dichlorosilane, and trichlorosilane. J Phys Chem A 105:2015–2022
Mohammadi MD, Abdullah HY, Biskos G, Bhowmick S (2021) Effect of Al-and Ga-doping on the adsorption of H2SiCl2 onto the outer surface of boron nitride nanotube: a DFT study. C R Chim 24:291–304
Mohammadi MD, Abdullah HY, Louis H, Mathias GE (2022) 2D Boron Nitride Material as a sensor for H2SiCl2. Comput Theor Chem 1213:113742
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465
Goerigk L, Grimme S (2011) A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. Phys Chem Chem Phys 13:6670–6688
Mardirossian N, Head-Gordon M (2017) Thirty years of density functional theory in computational chemistry: an overview and extensive assessment of 200 density functionals. Mol Phys 115:2315–2372
Brakestad A, Jensen SR, Wind P, D’Alessandro M, Genovese L, Hopmann KH, Frediani L (2020) Static polarizabilities at the basis set limit: A benchmark of 124 species. J Chem Theory Comput 16:4874–4882
Mitra H, Roy TK (2020) Comprehensive Benchmark results for the accuracy of basis sets for anharmonic molecular vibrations. J Phys Chem A 124:9203–9221
Goerigk L, Hansen A, Bauer C, Ehrlich S, Najibi A, Grimme S (2017) A look at the density functional theory zoo with the advanced GMTKN55 database for general main group thermochemistry, kinetics and noncovalent interactions. Phys Chem Chem Phys 19:32184–32215
Perdew JP, Ernzerhof M, Burke K (1996) Rationale for mixing exact exchange with density functional approximations. J Chem Phys 105:9982–9985
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: The PBE0 model. J Chem Phys 110:6158–6170
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theoret Chem Acc 120:215–241
Zhao Y, Truhlar DG (2006) Density functional for spectroscopy: no long-range self-interaction error, good performance for Rydberg and charge-transfer states, and better performance on average than B3LYP for ground states. J Phys Chem A 110:13126–13130
FrischMJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16 Rev. C.01, Wallingford, CT
Foster AJ, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102:7211–7218
Reed AE, Weinhold F (1983) Natural bond orbital analysis of near-Hartree–Fock water dimer. J Chem Phys 78:4066–4073
Carpenter J, Weinhold F (1988) Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J Mol Struct (Thoechem) 169:41–62
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
O’boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845
Mohammadi MD, Hamzehloo M (2018) The adsorption of bromomethane onto the exterior surface of aluminum nitride, boron nitride, carbon, and silicon carbide nanotubes: a PBC-DFT, NBO, and QTAIM study. Comput Theor Chem 1144:26–37
DoustMohammadi M, Abdullah HY (2020) The adsorption of chlorofluoromethane on pristine, Al-, Ga-, P-, and As-doped boron nitride nanotubes: a PBC-DFT, NBO, and QTAIM study. ChemistrySelect 5:12115–12124
Mohammadi MD, Salih IH, Abdullah HY (2020) The adsorption of chlorofluoromethane on pristine and Ge-doped silicon carbide nanotube: a PBC-DFT, NBO, and QTAIM study. Mol Simul 46:1405–1416
DoustMohammadi M, Abdullah HY (2021) The adsorption of bromochlorodifluoromethane on pristine and Ge-doped silicon carbide nanotube: a PBC-DFT, NBO, and QTAIM study. Struct Chem 32:481–494
Mohammadi MD, Abdullah HY (2021) The adsorption of bromochlorodifluoromethane on pristine, Al, Ga, P, and As-doped boron nitride nanotubes: a study involving PBC-DFT, NBO analysis, and QTAIM. Comput Theor Chem 1193:113047
Mohammadi MD, Abdullah HY (2021) Vinyl chloride adsorption onto the surface of pristine, Al-, and Ga-doped boron nitride nanotube: A DFT study. Solid State Commun 337:114440
Mohammadi MD, Abdullah HY (2021) DFT Study for Adsorbing of Bromine Monochloride onto BNNT (5, 5), BNNT (7, 0), BC 2 NNT (5, 5), and BC 2 NNT (7, 0). J Comput Biophys Chem 20:765–783
Mohammadi MD, Abdullah HY, Biskos G, Bhowmick S (2021) Enhancing the absorption of 1-chloro-1, 2, 2, 2-tetrafluoroethane on carbon nanotubes: an ab initio study. Bull Mater Sci 44:198
DoustMohammadi M, Abdullah HY (2022) Weak intermolecular interactions of cysteine on BNNT, BNAlNT and BC2NNT: a DFT investigation. Bull Mater Sci 45:33
Bredas J-L (2014) Mind the gap! Mater Horiz 1:17–19
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den einzelnen Elektronen eines Atoms. Physica 1:104–113
Janak J (1978) Proof that∂ e∂ n i= ε in density-functional theory. Phys Rev B 18:7165
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68:3801–3807
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516
Levy M (1982) Electron densities in search of Hamiltonians. Phys Rev A 26:1200
Baei MT, Peyghan AA, Bagheri Z (2012) A computational study of AlN nanotube as an oxygen detector. Chin Chem Lett 23:965–968
Yamazoe N, Shimanoe K (2009) New perspectives of gas sensor technology. Sens Actuators, B Chem 138:100–107
Hamprecht FA, Cohen AJ, Tozer DJ, Handy NC (1998) Development and assessment of new exchange-correlation functionals. J Chem Phys 109:6264–6271
Mulliken RS (1955) Electronic population analysis on LCAO–MO molecular wave functions I. J Chem Phys 23:1833–1840
Mayer I (1983) Charge, bond order and valence in the Ab initio SCF theory. Chem Phys Lett 97:270–274
Mayer I (2012) Improved definition of bond orders for correlated wave functions. Chem Phys Lett 544:83–86
Bridgeman AJ, Cavigliasso G, Ireland LR, Rothery J (2001) The Mayer bond order as a tool in inorganic chemistry. J Chem Soc Dalton Trans 2095–2108
Wiberg KB (1968) Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24:1083–1096
Sizova OV, Skripnikov LV, Sokolov AY (2008) Symmetry decomposition of quantum chemical bond orders. J Mol Struct (Thoechem) 870:1–9
Bader RF (1985) Atoms in molecules. Acc Chem Res 18:9–15
Bader R (1990) A quantum theory. Clarendon, Oxford
Bader RFW, Popelier PLA, Keith TA (1994) Theoretical definition of a functional group and the molecular orbital paradigm. Angew Chem Int Ed Engl 33:620–631
Matta CF (2006) Hydrogen–hydrogen bonding: the non-electrostatic limit of closed-shell interaction between two hydro, Hydrogen Bonding—New Insights, Springer, pp 337–375
Grabowski SJ (2012) QTAIM characteristics of halogen bond and related interactions. J Phys Chem A 116:1838–1845
Acknowledgements
HYA thanks the Solid-State Theory Group, Physics Department, Università degli Studi di Milano, Milan, Italy, for providing computational facilities. SB and GB acknowledge the support of the European Regional Development Fund and the Republic of Cyprus through the Research Innovation Foundation (Cy-Tera project under the grant NEA YPODOMH/STPATH/0308/31, and NANO2LAB project under the grant INFRASTRUCTURES/1216/0070).
Author information
Authors and Affiliations
Contributions
Mohsen Doust Mohammadi: Writing – original draft, Formal analysis. Hewa Y. Abdullah: Supervision, Investigation, Project administration. Somnath Bhowmick: Conceptualization, Validation. George Biskos: Resources, Visualization.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All authors agree.
Consent for Publication
All authors agree.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Research Involving Human Participants and/or Animals
Not applicable.
Informed Consent
All authors agree.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doust Mohammadi, M., Abdullah, H.Y., Bhowmick, S. et al. Silicon Carbide Based Nanotubes as a Sensing Material for Gaseous H2SiCl2. Silicon 15, 177–186 (2023). https://doi.org/10.1007/s12633-022-02010-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-022-02010-0