Abstract
Angiogenesis is essential for tumor growth and metastasis. Endocrine gland-derived vascular endothelial growth factor (EG-VEGF) is an angiogenic factor predominantly expressed in steroidogenic organs like the adrenal gland, ovary, testes, and placenta. EG-VEGF has antiapoptotic, mitogenic, and chemoattractive properties mediated via the two G protein-coupled receptors prokineticin receptor 1 (PKR1) and prokineticin receptor 2 (PKR2). We investigated the expression of EG-VEGF and its receptors in a large number of normal adrenal glands (NAG), adrenocortical adenomas (ACA), and carcinomas (ACC) using real-time PCR (NAG, n = 12; ACA, n = 24; and ACC, n = 30) and immunohistochemistry (NAG, n = 9; ACA, n = 23; and ACC, n = 163) and evaluated its impact on patients’ survival. EG-VEGF, PKR1, and PKR2 mRNA and protein are expressed in NAG and the vast majority of ACA and ACC samples. The mean EG-VEGF mRNA expression was significantly lower in ACC (606.5 ± 77.1 copies) compared to NAG (4,043 ± 1,111) and cortisol-producing adenomas (CPA) (4,433 ± 2,378) (p < 0.01 and p < 0.05, respectively). However, cytoplasmic and nuclear EG-VEGF protein expression was either significantly higher or similar in ACC (H score 2.4 ± 0.05, p < 0.05 and 1.7 ± 0.08, n.s., respectively) compared to NAG (1.8 ± 0.14 and 1.7 ± 0.2). Nuclear protein expression of either EG-VEGF or PKR1 or both is predictive for a higher mortality compared to patients without nuclear expression (hazard ratio (HR) = 5.15; 95 % confidence interval (CI) = 1.24–21.36, n = 100, p = 0.02 independent of age, sex, and tumor stage). These findings suggest that EG-VEGF and its receptor PKR1 might play a role in the pathogenesis of adrenocortical tumors and could serve as prognostic markers for this rare malignant disease.
Similar content being viewed by others
Introduction
Adrenocortical carcinoma (ACC) is a rare and highly malignant tumor whose pathogenesis is largely unclear [11, 13, 15]. Treatment options are limited and, beside surgery in localized stages, mitotane (adjuvantly or palliatively) or etoposide, doxorubicin, and cisplatin plus mitotane are the current standards [2, 14, 56]. Up to now, only a few prognostic markers are available to guide treatment decisions.
For several decades, it has been established that angiogenesis is essential for tumor growth and metastasis. It is impossible for tumors to expand for more than a few millimeters without neovascularization [21]. Antiangiogenic therapies, mostly targeting the angiogenic key factor vascular endothelial growth factor (VEGF) or its receptor VEGFR-2, are already successfully applied in many solid tumors such as colorectal carcinoma [26], renal cell carcinoma [40], neuroendocrine tumors [48], or thyroid cancer [5, 10, 59]. In 2001, the endocrine gland-derived VEGF (EG-VEGF) was identified as the first tissue-specific angiogenic factor predominantly expressed in steroidogenic organs like the adrenal gland, testes, ovary, and placenta. Both EG-VEGF and VEGF have a HIF-1 binding site and are induced by hypoxia. While sharing mitogenic, permeability enhancing, antiapoptotic, and chemoattractive properties, VEGF and EG-VEGF do not belong to the same gene family [31]. EG-VEGF (also known as prokineticin 1 or PK1) is a secreted glycoprotein and has prokinetic effects on gut [33]. EG-VEGF belongs to the AVIT protein family and shares the amino terminal sequence with prokineticin 2 (mammalian orthologue of Bombina variegata peptide 8), which is not expressed in human adrenal tissue [32]. The two G protein-coupled receptors prokineticin receptor 1 (PKR1) and prokineticin receptor 2 (PKR2) represent cognate receptors for EG-VEGF [36, 38]. EG-VEGF plays a role in the pathology of endocrine tumors, such as Leydig-cell-tumors [51], papillary thyroid cancer [47], and non-endocrine tumors like neuroblastoma [44], prostate cancer [46], gastrointestinal tumors [22, 23, 41, 55], pancreatic ductal adenocarcinoma [27, 39, 49], Merkel cell carcinoma [30], and multiple myeloma [34]. In bovine adrenal cortex-derived endothelial cells, EG-VEGF promotes proliferation, migration, and survival of responsive cells [36].
The adrenal gland is probably the highest vascularized organ in the body [42, 54]. Every adrenocyte is in contact with fenestrated endothelial cells ensuring sufficient oxygenation for hormone biosynthesis [57]. The expression of VEGF in ACC is well examined [1, 8, 29], but very little is known about EG-VEGF in adrenocortical tumors. Thus, we aimed to examine the expression of EG-VEGF, PKR1, and PKR2 in a large number of ACC, adrenocortical adenomas (ACA), and normal adrenal glands (NAG) using real-time PCR (NAG, n = 12; ACA, n = 24 (cortisol-producing adenoma, n = 8; aldosterone-producing adenoma, n = 8; endocrine-inactive adenoma, n = 8); and ACC, n = 30) and immunohistochemistry (NAG, n = 9; ACA, n = 23 (cortisol-producing adenoma, n = 8; aldosterone-producing adenoma, n = 8; endocrine-inactive adenoma, n = 7); and ACC, n = 163). Moreover, we evaluated its relationship with clinical data, including the impact on survival in ACC patients.
Materials and Methods
Clinical Data and Specimen
Tissue samples from NAG, ACA, and ACC were collected as described before [17]. Diagnosis was made based on clinical, laboratory, radiological, and pathological results. European Network for the Study of Adrenal Tumors (ENSAT) tumor stage (www.ensat.org) was used for the classification of ACC. Clinical data were collected by the German ACC Registry (www.nebennierenkarzinom.de) and through the European Network for the Study of Adrenal Tumors registry (www.ensat.org). Table 1 displays characteristics of patients and tumors. Patients gave informed consent for collecting tissue and clinical data, and the study was approved by the ethics committee of the University of Wuerzburg (Germany, board approval number 88/11).
RNA Extraction and Real-Time Quantitative PCR (qPCR)
RNA was extracted from frozen tumor tissue samples (30 ACC, 24 ACA, and 12 NAG) using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Reverse transcription was carried out using the iscript TM cDNA Synthesis Kit (Bio-Rad Laboratories GmbH, Munich, Germany) according to the manufacturer’s manual. Samples were diluted with aqua dest in a relation of 1:15 before use as a template.
Real-time quantitative PCR was performed in duplicates using the TaqMan Technology. A reaction mix of 20 μl containing distillated water, TaqMan MasterMix (Applied Biosystems, Darmstadt, Germany), and the primers/probe mixture in the relation 5:10:1 was added to 5 μl of cDNA (original RNA concentration, 3.31 ng/μl). Commercial probes were used (Applied Biosystems, 18s: Hs99999901_s1; EG-VEGF: Hs00951617_m1; PKR1: Hs00373446_m1; PKR2: Hs00431207_m1). A dilution series with a known cDNA copy number allowed absolute quantification of cDNA copy number for each sample.
Immunohistochemistry in Adrenocortical Tumor Samples
The immunohistochemical stainings were performed on a total of 195 adrenocortical tissue samples (163 ACC, 23 ACA, and nine NAG). The adrenal tumor samples were assembled into three tissue microarrays as described [17, 50]. Immunohistochemical detection was performed using an indirect immunoperoxidase technique following high temperature antigen retrieval in 0.01 M citrate buffer (pH 6.0). As primary antibodies (Table 2), we used EG-VEGF polyclonal rabbit antibody, dilution of 1:200, kindly provided by Elly S. W. Ngan, University of Hong Kong, PKR1 polyclonal rabbit antibody (GPR73A), dilution 1:150, and PKR2 polyclonal rabbit antibody (GPR 73 B), dilution 1:150 (both antibodies from MoBiTec (Molecular Biotechnology), Göttingen, Germany). The signal was developed using the DAKO HRP-System (DAKO, Copenhagen, Denmark) and NovaRed as substrate according to the manufacturer’s instructions (Vector Laboratories, Burlingame, USA). Nuclei were counterstained with hematoxylin. As a negative control, we employed an unspecific IgG isotype antibody and adrenal capsule adipose tissue as an internal control, and as a positive control, we used ovary tissue for EG-VEGF and prostate tissue for PKR1 and PKR2, showing specific cytoplasmatic staining in accordance with www.proteinatlas.org (supplementary Fig. 1). All tissue array slides were analyzed independently by two investigators (D.H. and L.K.). Samples were regarded as evaluable when at least two of five array spots were intact. Where discrepancies were observed, results were double checked by both investigators together with a third observer (S.S.). Cytoplasmic and nuclear staining intensity was assigned to the categories no staining (0), weak (1), moderate (2), and strong (3). The percentage of positive cells was assessed for each specimen and scored 0 if 0 % were positive, 0.5 if 10–49 %, and 1 if 50 % or more cells were positive. A semiquantitative H score was calculated by multiplying the staining intensity score with the percentage of positive cells score as described [45]. Later on, for survival analysis purpose, the weak, moderate, and strong stainings were accumulated into a general positive staining.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Differences in expression were analyzed using nonparametric Kruskal-Wallis test and Dunn’s post hoc test. Differences in PKR2 mRNA expression were analyzed using nonparametric Mann-Whitney test. We used a Cox regression model for overall survival analyses. Overall survival was defined as time elapsed from primary resection of ACC to death or last follow-up visit. A p value < 0.05 was regarded as significant. A univariate and an additional multivariate cox regression analysis including age, sex, and ENSAT tumor stage [12] was carried out. Statistical analyses were performed with SPSS statistics version 22 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism (version 6, GraphPad Software Inc., San Diego, CA, USA).
Results
EG-VEGF, PKR1, and PKR2 mRNA Expression
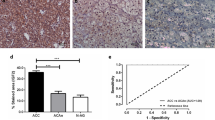
EG-VEGF mRNA was expressed in all NAG (n = 12), ACA (n = 24), and ACC (n = 30). Mean mRNA expression was highest in cortisol-producing adenomas (CPA, 4,433 ± 2,378 copies/16.55 ng RNA) similar to the expression in NAG (4,043 ± 1,111 copies). There was a significantly lower mRNA expression in ACC compared to NAG and CPA (606.5 ± 77 copies, p < 0.01 and p < 0.05, respectively) using ordinary one-way ANOVA with Turkey’s multiple comparisons test (Fig. 1a). The expression of PKR1 and PKR2 mRNA in NAG, ACA, and ACC was examined on a subset of samples. PKR1 mRNA could be detected in all five NAG (3,148 ± 2,842 copies), nine out of ten ACC (227.8 ± 42.93 copies), and four out of five ACA (two cortisol-producing adenomas, one aldosterone-producing adenoma, one endocrine-inactive adenoma) (2,301 ± 2,110 copies) with the strongest expression in the aldosterone-producing adenoma (8,630 copies) (Fig. 1b). PKR2 mRNA was expressed only very weakly (Fig. 1c).
EG-VEGF, PKR1, and PKR2 mRNA expression in adrenal tissues. EG-VEGF (a), PKR1 (b), and PKR2 (c) mRNA copy number/16.55 ng RNA is displayed for every sample. Black bars represent means with SEM. NAG normal adrenal glands, ACC adrenocortical carcinoma, ACA adrenocortical adenoma divided in cortisol-producing adenoma (CPA), aldosterone-producing adenoma (APA), and endocrine-inactive adenoma (EIA). *p < 0.05, **p < 0.01
EG-VEGF, PKR1, and PKR2 Protein Expression
Specificity of the antibodies was proven using positive and negative controls: The EG-VEGF antibody showed a specific staining on ovary tissue, and the PKR1 and PKR2 antibodies showed a specific staining on prostate tissue. Specific staining was detected in the cytoplasm, not in the nucleus. Employment of an unspecific IgG isotype antibody prevented positive staining, respectively. Moreover, on adrenal capsule tissue, no specific staining was detected (supplementary Fig. 1).
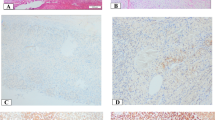
The immunohistochemical stainings of NAG, EG-VEGF, PKR1, and PKR2 revealed that these proteins were predominantly expressed in the adrenal cortex and only weakly or absent in the capsule and medulla. EG-VEGF expression was highest in the zona glomerulosa, whereas PKR1 and PKR2 proteins were equally detectable in the zona glomerulosa, zona fasciculata, and zona reticularis. The specific immunostaining was detected both in the nucleus and cytoplasm for all the three investigated proteins (Fig. 2 and Table 3). We therefore decided to evaluate the different cell compartments of each sample. The intra-tumor heterogeneity concerning cytoplasmatic and nuclear staining was predominantly minor (approximately 10 %). Hence, staining intensity and H scores were identical. Ninety-nine percent of the evaluable ACC showed a positive cytoplasmic staining against EG-VEGF with 51 % being strong (Table 3). EG-VEGF was also detectable in the cytoplasm of all NAG and ACA (Fig. 3a). Nuclear staining against EG-VEGF was present in 84 % of ACC, 91 % of ACA, and all NAG (Fig. 3b). PKR1 protein was expressed in the cytoplasm of 95 % of ACC, 89 % of NAG, and 95 % of ACA (Fig. 3c). Nuclear staining against PKR1, however, was only observed in 69 % of ACC, 77 % of NAG, and 68 % of ACA (Fig. 3d). In contrast, PKR2 protein staining was either negative or weak to moderate in all samples and independent of subcellular localization (Fig. 3e, f and Table 3). Cytoplasmic EG-VEGF expression was significantly higher in ACC (mean H score 2.4 ± 0.06) compared to NAG (mean H score 1.8 ± 0.14, p < 0.05). Apart from this, nuclear EG-VEGF expression and cytoplasmic and nuclear, PKR1, and PKR2 protein expression did not differ significantly between ACC, NAG, and ACA (Fig. 3).
Immunohistochemical staining of normal adrenal glands and adrenocortical carcinoma against EG-VEGF, PKR1, and PKR2. Expression of EG-VEGF (first row), PKR1 (second row), and PKR2 (third row) in normal adrenal glands (a, d, g, 1 = adrenal capsule, 2 = adrenal cortex (a = zona glomerulosa, b = zona fasciculata, c = zona reticularis), 3 = adrenal medulla) and ACC (b, c, e, f, h, i). b Example for positive nuclear staining. c Example for negative nuclear staining. Magnification: ×40
H score distribution of EG-VEGF, PKR1, and PKR2 immunohistochemical staining of normal and tumoral adrenocortical tissues. Summary of differential cytoplasmic (a, c, and e) and nuclear (b, d, and f) EG-VEGF (a and b), PKR1 (c and d), and PKR2 (e and f) staining intensity (H score) in normal adrenal glands (NAG), adrenocortical adenomas (ACA), and adrenocortical carcinomas (ACC)
Positive Nuclear Staining Against EG-VEGF and PKR1 Is Predictive for a Higher Mortality
We performed overall survival analyses using Cox regression plots only in ACC samples coming from primary tumors with available immunostaining and clinical data (for multivariate analysis: EG-VEGF n = 110, PKR1 n = 101, PKR2 n = 115) (Fig. 4 and Table 4). Cytoplasmic EG-VEGF, PKR1, and PKR2 expression did not correlate with overall survival. However, a positive nuclear staining against EG-VEGF was associated with a significantly higher mortality in patients with ACC (hazard ratio (HR) for death 2.78; 95 % confidence interval (CI) 1.27–6.08; p = 0.01) (Fig. 4a). Similarly, patients with a positive nuclear staining against PKR1 were more likely to die compared to patients with a negative nuclear expression of PKR1 (HR 2.22; 95 % CI 1.23–4.03; p < 0.01) (Fig. 4b). The prognostic value was even higher when either EG-VEGF or PKR1 protein or both were expressed in the nucleus of ACC cells compared to patients with none of these factors in the nucleus (HR 5.65; 95 % CI 1.38–23.12; p = 0.02) (Fig. 4c). Multivariate regression analysis including age, sex, and ENSAT tumor stage confirmed the independent prognostic value of this combination (EG-VEGF: HR 2.41, 95 % CI 1.08–5.38, p = 0.03; PKR1: HR 1.95, 95 % CI 1.06–3.56, p = 0.03; EG-VEGF or PKR1: HR 5.15, 95 % CI 1.24–21.36, p = 0.02) (Table 4). Excess of cortisol production did not influence survival in our patients (HR 1.06, CI 0.63–1.76, p = 0.84) (supplementary Fig. 2).
Univariate Cox regression survival curves based on nuclear expression of EG-VEGF and PKR1. a Survival of 112 patients with ACC depending on nuclear expression of EG-VEGF: negative nuclear EG-VEGF expression (grey) and positive (black). b Survival of 103 patients with ACC depending on nuclear expression of PKR1: negative nuclear PKR1 expression (grey) and positive (black). c Survival of 102 patients with ACC depending on nuclear expression of EG-VEGF and PKR1: both negative (grey) and both or at least one positive (black)
Discussion
The key finding of our study is the strong prognostic potential of nuclear staining of EG-VEGF and its receptor PKR1 for patient outcome in ACC. In general, EG-VEGF and both of its receptors PKR1 and PKR2 are present in most adrenocortical adenomas and carcinomas. However, only the nuclear localization harbors prognostic value. Indeed, we are the first to describe nuclear expression of the glycoprotein EG-VEGF and its G protein-coupled receptors PKR1 and PKR2. The specificity of our antibodies was proven on negative controls and positive controls in accordance with www.proteinatlas.org and previous publications on the expression of EG-VEGF in ovary tissue [18, 20] and PKR1 and PKR2 in prostate tissue [46]. The normal tissues showed in contrast to tumor tissues no nuclear staining. Over the last years, knowledge about intracellular protein transport has increased. It has been shown that subcellular trafficking of proteins to the “wrong” cell compartment, such as the nucleus for a membrane receptor, can result in disease like cancer by loss of function or gain of activity in the “wrong” cell compartment. Such nuclear misleading of proteins is known for epidermal growth factor receptor (EGFR) [4], FGF [37], and VEGF receptor [16]. Concerning EG-VEGF, this nuclear expression might represent active interaction of both ligand and receptor required for relevant influence on cell cycle and transcription. Remarkably, the EG-VEGF promotor has a potential binding site for an orphan nuclear receptor essential for adrenal development, steroidogenic-factor 1 (SF)-1, or NR5A1 [32]. In neuroblastoma cells, bovine adrenal cortex capillary endothelial cells and bovine glomerulosa and fasciculata cells, an autocrine proliferation mechanism of EG-VEGF, probably via its receptor PKR1, could be demonstrated [28, 35, 44]. Thus, we hypothesize that there exists a similar mechanism in tumor growth in ACC. Of note, if either EG-VEGF or PKR1 or both of them are present in the nucleus, the likelihood that patients die from ACC is more than five times higher than if none of these factors are detectable. Interestingly, this result is exactly confirmed in multivariate analysis.
Up to now, only the tumor stage is generally accepted as a prognostic tool. However, within a given tumor stage, survival of ACC patients is quite heterogeneous [25] resulting in uncertainty of clinicians regarding aggressiveness of treatment when confronted with an individual patient. Therefore, reliable prognostic markers are urgently needed. In the last years, few immunohistochemical markers with prognostic potential have been suggested [3, 9, 17, 52, 53, 58]. In addition to these markers, nuclear staining of EG-VEGF and its receptor PKR1 is interesting owing to its high prognostic value.
To our knowledge, this is the first report of the expression of EG-VEGF and its receptors PKR1 and PKR2 in a large number of NAG, ACA, and ACC. In our small mRNA study, EG-VEGF mRNA expression was significantly higher in NAG compared to ACC. Conversely, cytoplasmic EG-VEGF protein expression was significantly higher in ACC compared to NAG. There are multiple reasons for this discrepancy in mRNA and protein expression: Apart from the possible inaccuracy of technical methods and low sample size (only eight patients were identical in mRNA and protein analysis), mRNA expression does not always predict protein expression, especially in genes involved in development and regulation. Alternative splicing, translational modifications, and different degradation of mRNA and protein all have an impact on mRNA and protein quantities [24]. Besides the significant different EG-VEGF mRNA and cytoplasmic expression in ACC and NAG, the mRNA and protein expression of EG-VEGF, PKR1, and PKR2 did not show any significant differences between the adrenal entities. A possible explanation might be the existing strong vascularization of the normal adrenal gland as an endocrine organ [57], which is still present in adrenocortical tumors, although the vascular density in ACC is relatively lower than in NAG and ACA [1]. Moreover, angiogenesis is a highly complex process requiring the precise coordination of many angiogenic factors. Physiologic and pathologic angiogenesis are still not fully understood [6, 7, 19]. It is conceivable that the angiogenic factors EG-VEGF and VEGF interact in the adrenal gland as assumed by Thomas et al. [57].
The expression pattern of EG-VEGF, PKR1, and PKR2 protein in the adrenal cortex of NAG was different in human tissue compared to previous examinations by Keramidas et al. on bovine adrenal cortex tissue [28]. Both in human and bovine tissues, EG-VEGF, PKR1, and PKR2 were predominantly detectable in the cortex with only very weak, respectively, no, specific staining in the medulla or adrenal capsule. EG-VEGF expression was highest in the zona glomerulosa in human tissue, whereas EG-VEGF staining was slightly stronger in the zona fasciculata/reticularis in bovine tissue. PKR1 and PKR2 expression also differed among the two species with regard to a stronger expression of PKR2 in the bovine zona glomerulosa. A different expression pattern between the two species is in accordance with a previous study indicating a different expression of EG-VEGF among mammalian species, probably due to divergence in the promoter sequence [32].
Furthermore, EG-VEGF would be an interesting target for antiangiogenic therapies against ACC or tumors of the ovary and testes. In contrast to widespread VEGF, against which antiangiogenic therapies have already been successfully established, EG-VEGF is predominantly expressed in steroidogenic organs. Therefore, fewer side effects would be expected in anti-EG-VEGF therapies in comparison to anti-VEGF therapies. However, anti-EG-VEGF therapies might possibly cause gastrointestinal side effects given its prokinetic effect on gastrointestinal small muscle.
In summary, our work suggests an implication of EG-VEGF and its receptor PKR1 in pathogenesis of ACC. However, the most important finding is that nuclear staining of EG-VEGF together with PKR1 is one of the best prognostic markers for overall survival in patients with ACC.
References
Bernini GP, Moretti A, Bonadio AG, Menicagli M, Viacava P, Naccarato AG, Iacconi P, Miccoli P, Salvetti A (2002) Angiogenesis in human normal and pathologic adrenal cortex. J Clin Endocrinol Metab 87(11):4961–4965. doi:10.1210/jc.2001-011799
Berruti A, Baudin E, Gelderblom H, Haak HR, Porpiglia F, Fassnacht M, Pentheroudakis G (2012) Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):vii131–138. doi:10.1093/annonc/mds231
Beuschlein F, Weigel J, Saeger W, Kroiss M, Wild V, Daffara F, Libe R et al (2015) Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab 100(3):841–849. doi:10.1210/jc.2014-3182
Bitler BG, Goverdhan A, Schroeder JA (2010) MUC1 regulates nuclear localization and function of the epidermal growth factor receptor. J Cell Sci 123(Pt 10):1716–1723. doi:10.1242/jcs.062661
Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C et al (2014) Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384(9940):319–328. doi:10.1016/s0140-6736(14)60421-9
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438(7070):932–936. doi:10.1038/nature04478
Coultas L, Chawengsaksophak K, Rossant J (2005) Endothelial cells and VEGF in vascular development. Nature 438(7070):937–945. doi:10.1038/nature04479
de Fraipont F, El Atifi M, Gicquel C, Bertagna X, Chambaz EM, Feige JJ (2000) Expression of the angiogenesis markers vascular endothelial growth factor-A, thrombospondin-1, and platelet-derived endothelial cell growth factor in human sporadic adrenocortical tumors: correlation with genotypic alterations. J Clin Endocrinol Metab 85(12):4734–4741. doi:10.1210/jcem.85.12.7012
Duregon E, Volante M, Giorcelli J, Terzolo M, Lalli E, Papotti M (2013) Diagnostic and prognostic role of steroidogenic factor 1 in adrenocortical carcinoma: a validation study focusing on clinical and pathologic correlates. Hum Pathol 44(5):822–828. doi:10.1016/j.humpath.2012.07.025
Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, Licitra L et al (2013) Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31(29):3639–3646. doi:10.1200/jco.2012.48.4659
Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD (2014) Adrenocortical carcinoma. Endocr Rev 35(2):282–326. doi:10.1210/er.2013-1029
Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH, Hahner S, Allolio B (2009) Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer 115(2):243–250. doi:10.1002/cncr.24030
Fassnacht M, Kroiss M, Allolio B (2013) Update in adrenocortical carcinoma. J Clin Endocrinol Metab 98(12):4551–4564. doi:10.1210/jc.2013-3020
Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S et al (2012) Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med 366(23):2189–2197. doi:10.1056/NEJMoa1200966
Fay AP, Elfiky A, Telo GH, McKay RR, Kaymakcalan M, Nguyen PL, Vaidya A, Ruan DT, Bellmunt J, Choueiri TK (2014) Adrenocortical carcinoma: the management of metastatic disease. Crit Rev Oncol Hematol. doi:10.1016/j.critrevonc.2014.05.009
Feng Y, Venema VJ, Venema RC, Tsai N, Caldwell RB (1999) VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochem Biophys Res Commun 256(1):192–197. doi:10.1006/bbrc.1998.9790
Fenske W, Volker HU, Adam P, Hahner S, Johanssen S, Wortmann S, Schmidt M et al (2009) Glucose transporter GLUT1 expression is an stage-independent predictor of clinical outcome in adrenocortical carcinoma. Endocr Relat Cancer 16(3):919–928. doi:10.1677/erc-08-0211
Ferrara N, Frantz G, LeCouter J, Dillard-Telm L, Pham T, Draksharapu A, Giordano T, Peale F (2003) Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am J Pathol 162(6):1881–1893. doi:10.1016/S0002-9440(10)64322-2
Ferrara N, Kerbel RS (2005) Angiogenesis as a therapeutic target. Nature 438(7070):967–974. doi:10.1038/nature04483
Fraser HM, Bell J, Wilson H, Taylor PD, Morgan K, Anderson RA, Duncan WC (2005) Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. J Clin Endocrinol Metab 90(1):427–434. doi:10.1210/jc.2004-0843
Gimbrone MA Jr, Leapman SB, Cotran RS, Folkman J (1972) Tumor dormancy in vivo by prevention of neovascularization. J Exp Med 136(2):261–276
Goi T, Fujioka M, Satoh Y, Tabata S, Koneri K, Nagano H, Hirono Y, Katayama K, Hirose K, Yamaguchi A (2004) Angiogenesis and tumor proliferation/metastasis of human colorectal cancer cell line SW620 transfected with endocrine glands-derived-vascular endothelial growth factor, as a new angiogenic factor. Cancer Res 64(6):1906–1910
Goi T, Nakazawa T, Hirono Y, Yamaguchi A (2013) Prokineticin 1 expression in gastrointestinal tumors. Anticancer Res 33(12):5311–5315
Guo Y, Xiao P, Lei S, Deng F, Xiao GG, Liu Y, Chen X et al (2008) How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin (Shanghai) 40(5):426–436
Hermsen IG, Gelderblom H, Kievit J, Romijn JA, Haak HR (2008) Extremely long survival in six patients despite recurrent and metastatic adrenal carcinoma. Eur J Endocrinol 158(6):911–919. doi:10.1530/eje-07-0723
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691
Jiang X, Abiatari I, Kong B, Erkan M, De Oliveira T, Giese NA, Michalski CW, Friess H, Kleeff J (2009) Pancreatic islet and stellate cells are the main sources of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 in pancreatic cancer. Pancreatology 9(1–2):165–172. doi:10.1159/000178888
Keramidas M, Faudot C, Cibiel A, Feige JJ, Thomas M (2008) Mitogenic functions of endocrine gland-derived vascular endothelial growth factor and Bombina variegata 8 on steroidogenic adrenocortical cells. J Endocrinol 196(3):473–482. doi:10.1677/JOE-07-0255
Kroiss M, Reuss M, Kuhner D, Johanssen S, Beyer M, Zink M, Hartmann MF et al (2011) Sunitinib inhibits cell proliferation and alters steroidogenesis by down-regulation of HSD3B2 in adrenocortical carcinoma cells. Front Endocrinol (Lausanne) 2:27. doi:10.3389/fendo.2011.00027
Lauttia S, Sihto H, Kavola H, Koljonen V, Bohling T, Joensuu H (2014) Prokineticins and Merkel cell polyomavirus infection in Merkel cell carcinoma. Br J Cancer 110(6):1446–1455. doi:10.1038/bjc.2014.20
LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G et al (2001) Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 412(6850):877–884. doi:10.1038/35091000
LeCouter J, Lin R, Frantz G, Zhang Z, Hillan K, Ferrara N (2003) Mouse endocrine gland-derived vascular endothelial growth factor: a distinct expression pattern from its human ortholog suggests different roles as a regulator of organ-specific angiogenesis. Endocrinology 144(6):2606–2616. doi:10.1210/en.2002-0146
Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY (2001) Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol 59(4):692–698
Li QF, Zhu HY, Yang YF, Liu J, Xiao FJ, Zhang QW, Wu CT, Wang H, Wang LS (2010) Prokineticin-1/endocrine gland-derived vascular endothelial growth factor is a survival factor for human multiple myeloma cells. Leuk Lymphoma 51(10):1902–1912. doi:10.3109/10428194.2010.512963
Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY (2002) Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem 277(22):19276–19280. doi:10.1074/jbc.M202139200
Lin R, LeCouter J, Kowalski J, Ferrara N (2002) Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J Biol Chem 277(10):8724–8729. doi:10.1074/jbc.M110594200
Maher PA (1996) Nuclear translocation of fibroblast growth factor (FGF) receptors in response to FGF-2. J Cell Biol 134(2):529–536
Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M et al (2002) Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Commun 293(1):396–402. doi:10.1016/S0006-291X(02)00239-5
Morales A, Vilchis F, Chavez B, Chan C, Robles-Diaz G, Diaz-Sanchez V (2007) Expression and localization of endocrine gland-derived vascular endothelial growth factor (EG-VEGF) in human pancreas and pancreatic adenocarcinoma. J Steroid Biochem Mol Biol 107(1–2):37–41. doi:10.1016/j.jsbmb.2007.02.006
Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS et al (2006) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24(1):16–24. doi:10.1200/jco.2005.02.2574
Nagano H, Goi T, Koneri K, Hirono Y, Katayama K, Yamaguchi A (2007) Endocrine gland-derived vascular endothelial growth factor (EG-VEGF) expression in colorectal cancer. J Surg Oncol 96(7):605–610. doi:10.1002/jso.20716
Neuman KO (1912) The oxygen exchange of the suprarenal gland. J Physiol 45(3):188–196
Ngan ES, Lee KY, Yeung WS, Ngan HY, Ng EH, Ho PC (2006) Endocrine gland-derived vascular endothelial growth factor is expressed in human peri-implantation endometrium, but not in endometrial carcinoma. Endocrinology 147(1):88–95. doi:10.1210/en.2005-0543
Ngan ES, Sit FY, Lee K, Miao X, Yuan Z, Wang W, Nicholls JM et al (2007) Implications of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 signaling in human neuroblastoma progression. Clin Cancer Res 13(3):868–875. doi:10.1158/1078-0432.CCR-06-2176
Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, Taranchon E et al (2006) DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 355(10):983–991. doi:10.1056/NEJMoa060570
Pasquali D, Rossi V, Staibano S, De Rosa G, Chieffi P, Prezioso D, Mirone V et al (2006) The endocrine-gland-derived vascular endothelial growth factor (EG-VEGF)/prokineticin 1 and 2 and receptor expression in human prostate: up-regulation of EG-VEGF/prokineticin 1 with malignancy. Endocrinology 147(9):4245–4251. doi:10.1210/en.2006-0614
Pasquali D, Santoro A, Bufo P, Conzo G, Deery WJ, Renzullo A, Accardo G, Sacco V, Bellastella A, Pannone G (2011) Upregulation of endocrine gland-derived vascular endothelial growth factor in papillary thyroid cancers displaying infiltrative patterns, lymph node metastases, and BRAF mutation. Thyroid 21(4):391–399. doi:10.1089/thy.2010.0168
Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J et al (2011) Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364(6):501–513. doi:10.1056/NEJMoa1003825
Ren LN, Li QF, Xiao FJ, Yan J, Yang YF, Wang LS, Guo XZ, Wang H (2009) Endocrine glands-derived vascular endothelial growth factor protects pancreatic cancer cells from apoptosis via upregulation of the myeloid cell leukemia-1 protein. Biochem Biophys Res Commun 386(1):35–39. doi:10.1016/j.bbrc.2009.05.149
Ronchi CL, Sbiera S, Kraus L, Wortmann S, Johanssen S, Adam P, Willenberg HS, Hahner S, Allolio B, Fassnacht M (2009) Expression of excision repair cross complementing group 1 and prognosis in adrenocortical carcinoma patients treated with platinum-based chemotherapy. Endocr Relat Cancer 16(3):907–918. doi:10.1677/ERC-08-0224
Samson M, Peale FV Jr, Frantz G, Rioux-Leclercq N, Rajpert-De Meyts E, Ferrara N (2004) Human endocrine gland-derived vascular endothelial growth factor: expression early in development and in Leydig cell tumors suggests roles in normal and pathological testis angiogenesis. J Clin Endocrinol Metab 89(8):4078–4088. doi:10.1210/jc.2003-032024
Sbiera S, Schmull S, Assie G, Voelker HU, Kraus L, Beyer M, Ragazzon B et al (2010) High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J Clin Endocrinol Metab 95(10):E161–171. doi:10.1210/jc.2010-0653
Soon PS, Tacon LJ, Gill AJ, Bambach CP, Sywak MS, Campbell PR, Yeh MW et al (2009) miR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clin Cancer Res 15(24):7684–7692. doi:10.1158/1078-0432.ccr-09-1587
Stewart GN (1917) A note on some obvious consequences of the high rate of blood flow through the adrenals. Am J Physiol 45(1):92–95
Tabata S, Goi T, Nakazawa T, Kimura Y, Katayama K, Yamaguchi A (2013) Endocrine gland-derived vascular endothelial growth factor strengthens cell invasion ability via prokineticin receptor 2 in colon cancer cell lines. Oncol Rep 29(2):459–463. doi:10.3892/or.2012.2124
Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R et al (2007) Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med 356(23):2372–2380. doi:10.1056/NEJMoa063360
Thomas M, Keramidas M, Monchaux E, Feige JJ (2003) Role of adrenocorticotropic hormone in the development and maintenance of the adrenal cortical vasculature. Microsc Res Tech 61(3):247–251. doi:10.1002/jemt.10333
Volante M, Bollito E, Sperone P, Tavaglione V, Daffara F, Porpiglia F, Terzolo M, Berruti A, Papotti M (2009) Clinicopathological study of a series of 92 adrenocortical carcinomas: from a proposal of simplified diagnostic algorithm to prognostic stratification. Histopathology 55(5):535–543. doi:10.1111/j.1365-2559.2009.03423.x
Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E et al (2012) Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30(2):134–141. doi:10.1200/jco.2011.35.5040
Acknowledgments
This study was supported by grants from the Deutsche Forschungsgemeinschaft (grant FA 466/4-1 to M.F.), the Wilhelm Sander-Stiftung (grant 2012.095.2 to M.F.), and the Bayer AG (Grants4targets 2014-03-1075 to S.S.).
We thank Elly S. W. Ngan, University of Hong Kong, for kindly providing the EG-VEGF polyclonal rabbit antibody [43].
We also greatly appreciate the IT support for the German ACC Registry (Uwe Maeder, Wuerzburg, Germany) and for the European ENSAT ACC Registry (Anthony Stell, Melbourne, Australia). Without their continuous support, the detailed clinical data used here would not have been impossible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.

Supplementary Fig. 1
Positive and negative controls for EG-VEGF-, PKR1-, and PKR2-antibodies. Displayed are the positive (a, c, e) and negative controls (b, d, f) for EG-VEGF, PKR1, and PKR2-antibodies, respectively. A: ovary tissue showing specific cytoplasmatic staining against EG-VEGF. C: prostate tissue showing specific cytoplasmatic staining against PKR1. E: prostate tissue showing specific cytoplasmatic staining against PKR2. B, D, F: negative controls with employment of an unspecific IgG isotype antibody to B: ovary tissue, D and F: prostate tissue. Magnification: ×10, small boxes ×40. (JPEG 2,327 kb)

Supplementary Fig. 2
Univariate Cox regression survival curves based on excess cortisol production. Survival of 84 patients with ACC depending on excess cortisol production (+/− other hormones) (black), n = 48, and no excess of cortisol (grey), n = 36. (JPEG 552 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Heck, D., Wortmann, S., Kraus, L. et al. Role of Endocrine Gland-Derived Vascular Endothelial Growth Factor (EG-VEGF) and Its Receptors in Adrenocortical Tumors. HORM CANC 6, 225–236 (2015). https://doi.org/10.1007/s12672-015-0236-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-015-0236-z