Abstract
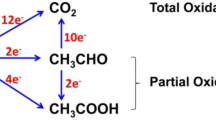
Electrocatalysts composed of Pt, and combined RhPt and RuRhPt sub-monolayers deposited on Au/C nanoparticles, were investigated for the electro-oxidation of ethanol. The Au/C substrates were characterized by transmission electron microscopy (TEM) and X-ray absorption near edge structure (XANES) in situ. The electrochemical activity and the products of the ethanol electro-oxidation were investigated by online differential electrochemical mass spectrometry (DEMS). TEM analysis of the Au/C substrate indicated a homogeneous dispersion of the Au atoms onto the carbon support, with particle sizes varying from 5 to 10 nm. The XANES results have evidenced lower increase of the Pt white line magnitude for the mixed layers as the electrode potential was increased. This was associated to a lower oxide formation at the Pt surface due to the presence of neighboring Rh and Ru atoms. Electrochemical stripping of adsorbed CO showed higher activities for the mixed layers as compared to the pure Pt layer and to Pt/C. The electrochemical results for the ethanol electro-oxidation evidenced very similar onset potential for the mixed sub-monolayers and Pt/C. DEMS measurements pointed out a negligible signal of CO2 for the pure Pt layer but an efficiency improvement for the CO2 formation when Rh was present on the electrocatalyst surface. The deposition of a second Pt layer induced a slight increase in the electrocatalyst activity and CO2 formation, which approached to that of Pt/C. It was demonstrated that the electrocatalyst efficiency can be hampered by optimizing the particle shell composition.











Similar content being viewed by others
References
Iwasita T, Pastor E (1994) A DEMS and FTir spectroscopic investigation of adsorbed ethanol on polycrystalline platinum. Electrochim Acta 39:531–537
de Souza JPI, Queiroz SL, Bergamaski K, Gonzalez ER, Nart FC (2002) Electro-oxidation of ethanol on Pt, Rh, and PtRh electrodes. A study using DEMS and in-situ FTIR techniques. J Phys Chem B 106:9825–9830
Vielstich W, Gasteiger HA, Lamm A (2003) In: Handbook of fuel cells—fundamentals, technology and applications. Wiley, Chichester
Camara GA, de Lima RB, Iwasita T (2005) The influence of PtRu atomic composition on the yields of ethanol oxidation: a study by in situ FTIR spectroscopy. J Electroanal Chem 585:128–131
Camara GA, Iwasita T (2005) Parallel pathways of ethanol oxidation: the effect of ethanol concentration. J Electroanal Chem 578:315–321
Lamy C, Rousseau S, Belgsir EM, Coutanceau C, Leger J-M (2004) Recent progress in the direct ethanol fuel cell: development of new platinum–tin electrocatalysts. Electrochim Acta 49:3901–3908
Leger J-M, Rousseau S, Coutanceau C, Hahn F, Lamy C (2005) How bimetallic electrocatalysts does work for reactions involved in fuel cells?: example of ethanol oxidation and comparison to methanol. Electrochim Acta 50:5118–5125
Lima FHB, Gonzalez ER (2008) Ethanol electro-oxidation on carbon-supported Pt–Ru, Pt–Rh and Pt–Ru–Rh nanoparticles. Electrochim Acta 53:2963–2971
Lima FHB, Profeti D, Lizcano-Valbuena W, Ticianelli EA, Gonzalez ER (2008) Carbon-dispersed Pt–Rh nanoparticles for ethanol electro-oxidation. Effect of the crystallite size and of temperature. J Electroanal Chem 617/2:121–129
Zhang J, Mo Y, Vukmirovic MB, Klie R, Sasaki K, Adzic RR (2004) Platinum monolayer electrocatalysts for O2 reduction: Pt monolayer on Pd(111) and on carbon-supported Pd nanoparticles. J Phys Chem B 108:10955–10964
Zhang J, Lima FHB, Shao MH, Sasaki K, Wang JX, Hanson J, Adzic RR (2005) Platinum monolayer on nonnoble metal–noble metal core–shell nanoparticle electrocatalysts for O2 reduction. J Phys Chem B 109:22701–22704
Hammer B, Nørskov JK (1995) Electronic factors determining the reactivity of metal surfaces. Surf Sci 343:211–220
Kitchin JR, Nørskov JK, Barteau MA, Chen G (2004) Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3 d transition metals. J Chem Phys 120:10240
Hammer B, Nørskov JK (2000) Theoretical surface science and catalysis—calculations and concepts. Adv Catal 45:71–129
Greeley J, Nørskov JK, Mavrikakis M (2002) Electronic structure and catalysis on metal surfaces. Annu Rev Phys Chem 53:319–348
Kristian N, Wang X (2008) Ptshell–Aucore/C electrocatalyst with a controlled shell thickness and improved Pt utilization for fuel cell reactions. Electrochem Commun 10:12–15
Schmidt TJ, Gasteiger HA, Stäb GD, Urban PM, Kolb DM, Behm RJ (1998) Characterization of high-surface-area electrocatalysts using a rotating disk electrode configuration. J Electrochem Soc 145:2354–2358
de Souza JPI, Queiroz SL, Nart FC (2000) The use of mass spectrometry in electrochemical measurements—the DEMS technique. Quim Nova 23(3):384–391
Bittins-Cattaneo B, Cattaneo E, Konigshoven P, Vielstich W (1991) In: Bard AJ (ed) Electroanalytical chemistry—a series of advances, vol 17. Marcel Dekker, New York, p 181
Ianniello R, Ber. Schmidt VM (1995) Simplified DEMS set up for electrocatalytic studies of porous PtRu alloys. Bunsen-Ges Phys Chem 99:83
Souza JPI, Iwasita T, Nart FC, Vielstich W (1999) Performance evaluation of porous electrocatalysts via normalization of the active surface. J Appl Electrochem 30:43–48
Lima FHB, de Castro JFR, Santos LGRA, Ticianelli EA (2009) Electrocatalysis of oxygen reduction on carbon-supported Pt-co nanoparticles with low Pt content. J Power Souces 190:293–300
McBreen J, O’Grady WE, Pandya KI, Roffman RW, Sayers DE (1987) EXAFS study of the nickel oxide electrode. Langumuir 3:428–433
Pandya KI, Roffman RW, McBreen J, O’Grady WE (1990) In situ x-ray absorption spectroscopic studies of nickel oxide electrodes. J Electrochem Soc 137:383–388
van Zon JBAC, Konigsberger DC, Van’t Blik HFJ, Sayers DE (1985) An EXAFS study of the structure of the metal–support interface in highly dispersed Rh/Al2O3 catalysts. J Chem Phys 82:5742
Sasaki K, Wang JX, Naohara H, Marinkovic N, More K, Inada H, Adzic RR (2010) Recent advances in platinum monolayer electrocatalysts for oxygen reduction reaction: scale-up synthesis, structure and activity of Pt shells on Pd cores. Electrochim Acta 55:2645–2652
Shao M, Sasaki K, Marinkovic NS, Zhang L, Adzic RR (2007) Synthesis and characterization of platinum monolayer oxygen-reduction electrocatalysts with Co–Pd core–shell nanoparticle supports. Electrochem Comm 9:2848–2853
Adzic RR, Lima FHB (2009) In: Vielstich W, Yokokawa H, Gasteiger HA (eds) Handbook of fuel cells, fundamentals, technology and applications, vol. 5. Wiley, p 5
Angersteinkozlowska H, Conway BE, Hamelin A, Stoicoviciu L (1987) Elementary steps of electrochemical oxidation of single-crystal planes of Au Part II. A chemical and structural basis of oxidation of the (111) plane. J Electroanal Chem 228:429–453
Mrozek MF, Xie Y, Weaver MJ (2001) Surface-enhanced raman scattering on uniform platinum-group overlayers: preparation by redox replacement of underpotential-deposited metals on gold. Anal Chem 73:5953–5960
Yu Y, Hu Y, Liu X, Deng W, Wang X (2009) The study of Pt@Au electrocatalyst based on Cu underpotential deposition and Pt redox replacement. Electrochim Acta 54:3092–3097
Manne S, Hansma PK, Massie J, Elings VB, Gewirth AA (1991) Atomic-resolution electrochemistry with the atomic force microscope: copper deposition on gold. Science 251:183–186
Martinez-Ruiz A, Palomar-Pardave M, Batina N (2008) Overpotential deposition of copper on an iodine-modified Au(111) electrode. Electrochimica Acta 53:2115–2120
Gasteiger HA, Markovic N, Ross PN Jr, Cairns EJ (1994) Carbon monoxide electrooxidation on well-characterized platinum-ruthenium alloys. J Phys Chem 98:617–625
Gupta SS, Datta J (2006) A comparative study on ethanol oxidation behavior at Pt and PtRh electrodeposits. J Electroanal Chem 594:65–72
Mukerjee S, Srinivasan S, Soriaga MP, McBreen J (1995) Role of structural and electronic properties of Pt and Pt alloys on electrocatalysis of oxygen reduction. J Electrochem Soc 142:1409–1422
Watanabe M, Motoo S (1975) Electrocatalysis by ad-atoms: Part II. Enhancement of the oxidation of methanol on platinum by ruthenium ad-atoms. J Electroanal Chem 60:267–273
Maillard F, Lu G-Q, Wiechowiski A, Stimming U (2005) Ru-decorated Pt surfaces as model fuel cell electrocatalysts for CO electrooxidation. J Phys Chem B 109:16230–16243
Maillard F, Savinova ER, Stimming U (2007) CO monolayer oxidation on Pt nanoparticles: further insights into the particle size effects. J Electroanal Chem 599:221–232
Acknowledgments
The authors thank the Fundação de Amparo à Pesquisa do Estado de São Paulo for financial support, the Brazilian Synchrotron Light Laboratory (LNLS) for the XAS experiments, and the MINATEC, Grenoble, for the TEM measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lima, F.H.B., Profeti, D., Chatenet, M. et al. Electro-oxidation of Ethanol on Rh/Pt and Ru/Rh/Pt Sub-monolayers Deposited on Au/C Nanoparticles. Electrocatal 1, 72–82 (2010). https://doi.org/10.1007/s12678-010-0014-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-010-0014-1