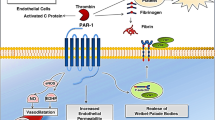
Abstract
Thrombin is increased in the brain after hemorrhagic and ischemic stroke primarily due to the prothrombin entry from blood either with a hemorrhage or following blood-brain barrier disruption. Increasing evidence indicates that thrombin and its receptors (protease-activated receptors (PARs)) play a major role in brain pathology following ischemic and hemorrhagic stroke (including intracerebral, intraventricular, and subarachnoid hemorrhage). Thrombin and PARs affect brain injury via multiple mechanisms that can be detrimental or protective. The cleavage of prothrombin into thrombin is the key step of hemostasis and thrombosis which takes place in every stroke and subsequent brain injury. The extravascular effects and direct cellular interactions of thrombin are mediated by PARs (PAR-1, PAR-3, and PAR-4) and their downstream signaling in multiple brain cell types. Such effects include inducing blood-brain-barrier disruption, brain edema, neuroinflammation, and neuronal death, although low thrombin concentrations can promote cell survival. Also, thrombin directly links the coagulation system to the immune system by activating interleukin-1α. Such effects of thrombin can result in both short-term brain injury and long-term functional deficits, making extravascular thrombin an understudied therapeutic target for stroke. This review examines the role of thrombin and PARs in brain injury following hemorrhagic and ischemic stroke and the potential treatment strategies which are complicated by their role in both hemostasis and brain.




Similar content being viewed by others
References
Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30(43):10363–70. https://doi.org/10.1021/bi00107a001.
Dihanich M, Kaser M, Reinhard E, Cunningham D, Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron. 1991;6(4):575–81. https://doi.org/10.1016/0896-6273(91)90060-d.
Weinstein JR, Gold SJ, Cunningham DD, Gall CM. Cellular localization of thrombin receptor mRNA in rat brain: expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. J Neurosci. 1995;15(4):2906–19.
Jamison CS, Degen SJ. Prenatal and postnatal expression of mRNA coding for rat prothrombin. Biochim Biophys Acta. 1991;1088(2):208–16. https://doi.org/10.1016/0167-4781(91)90056-r.
Maggio N, Itsekson Z, Dominissini D, Blatt I, Amariglio N, Rechavi G, et al. Thrombin regulation of synaptic plasticity: implications for physiology and pathology. Exp Neurol. 2013;247:595–604. https://doi.org/10.1016/j.expneurol.2013.02.011.
Ben Shimon M, Lenz M, Ikenberg B, Becker D, Shavit Stein E, Chapman J, et al. Thrombin regulation of synaptic transmission and plasticity: implications for health and disease. Front Cell Neurosci. 2015;9:151. https://doi.org/10.3389/fncel.2015.00151.
Rajput PS, Lamb J, Kothari S, Pereira B, Soetkamp D, Wang Y, et al. Neuron-generated thrombin induces a protective astrocyte response via protease activated receptors. Glia. 2020;68(2):246–62. https://doi.org/10.1002/glia.23714.
Sokolova E, Reiser G. Prothrombin/thrombin and the thrombin receptors PAR-1 and PAR-4 in the brain: localization, expression and participation in neurodegenerative diseases. Thromb Haemost. 2008;100(4):576–81.
Lee KR, Betz AL, Kim S, Keep RF, Hoff JT. The role of the coagulation cascade in brain edema formation after intracerebral hemorrhage. Acta Neurochir (Wien). 1996;138(4):396–400; discussion −1. https://doi.org/10.1007/bf01420301.
Keep RF, Xiang J, Ennis SR, Andjelkovic A, Hua Y, Xi G, et al. Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochir Suppl. 2008;105:73–7. https://doi.org/10.1007/978-3-211-09469-3_15.
Chen B, Cheng Q, Yang K, Lyden PD. Thrombin mediates severe neurovascular injury during ischemia. Stroke. 2010;41(10):2348–52. https://doi.org/10.1161/STROKEAHA.110.584920.
Krenzlin H, Lorenz V, Danckwardt S, Kempski O, Alessandri B. The importance of thrombin in cerebral injury and disease. Int J Mol Sci. 2016;17(1):84. https://doi.org/10.3390/ijms17010084.
Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc Natl Acad Sci U S A. 2000;97(5):2264–9. https://doi.org/10.1073/pnas.040552897.
Xi G, Keep RF, Hua Y, Xiang J, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30(6):1247–55. https://doi.org/10.1161/01.str.30.6.1247.
Bao X, Hua Y, Keep RF, Xi G. Thrombin-induced tolerance against oxygen-glucose deprivation in astrocytes: role of protease-activated receptor-1. Conditioning medicine. 2018;1(2):57–63.
Lee KR, Kawai N, Kim S, Sagher O, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86(2):272–8. https://doi.org/10.3171/jns.1997.86.2.0272.
Xi G, Wagner KR, Keep RF, Hua Y, de Courten-Myers GM, Broderick JP, et al. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998;29(12):2580–6. https://doi.org/10.1161/01.str.29.12.2580.
Hua Y, Wu J, Keep RF, Hoff JT, Xi G. Thrombin exacerbates brain edema in focal cerebral ischemia. Acta Neurochir Suppl. 2003;86:163–6. https://doi.org/10.1007/978-3-7091-0651-8_34.
Vaughan PJ, Pike CJ, Cotman CW, Cunningham DD. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. J Neurosci. 1995;15(7 Pt 2):5389–401.
Thevenet J, Angelillo-Scherrer A, Price M, Hirt L. Coagulation factor Xa activates thrombin in ischemic neural tissue. J Neurochem. 2009;111(3):828–36. https://doi.org/10.1111/j.1471-4159.2009.06369.x.
Fenton JW 2nd. Thrombin. Ann N Y Acad Sci. 1986;485:5–15. https://doi.org/10.1111/j.1749-6632.1986.tb34563.x.
Delvaeye M, Conway EM. Coagulation and innate immune responses: can we view them separately? Blood. 2009;114(12):2367–74. https://doi.org/10.1182/blood-2009-05-199208.
Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J Neurochem. 2003;84(1):3–9. https://doi.org/10.1046/j.1471-4159.2003.01268.x.
Burzynski LC, Humphry M, Pyrillou K, Wiggins KA, Chan JNE, Figg N, et al. The coagulation and immune systems are directly linked through the activation of interleukin-1alpha by thrombin. Immunity. 2019;50(4):1033–42.e6. https://doi.org/10.1016/j.immuni.2019.03.003.
Petzold T, Massberg S. Thrombin: a gas pedal driving innate immunity. Immunity. 2019;50(4):1024–6. https://doi.org/10.1016/j.immuni.2019.03.006.
van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87(4):335–40. https://doi.org/10.1161/01.res.87.4.335.
Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393(10175):1021–32. https://doi.org/10.1016/S0140-6736(19)30195-3.
Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. https://doi.org/10.1038/ncomms2230.
Ryu JK, Petersen MA, Murray SG, Baeten KM, Meyer-Franke A, Chan JP, et al. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat Commun. 2015;6:8164. https://doi.org/10.1038/ncomms9164.
Petersen MA, Ryu JK, Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci. 2018;19(5):283–301. https://doi.org/10.1038/nrn.2018.13.
Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–64. https://doi.org/10.1038/35025229.
Carney DH, Cunningham DD. Role of specific cell surface receptors in thrombin-stimulated cell division. Cell. 1978;15(4):1341–9. https://doi.org/10.1016/0092-8674(78)90059-4.
Wang H, Ubl JJ, Reiser G. Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia. 2002;37(1):53–63. https://doi.org/10.1002/glia.10012.
Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84(2):579–621. https://doi.org/10.1152/physrev.00028.2003.
Posma JJ, Posthuma JJ, Spronk HM. Coagulation and non-coagulation effects of thrombin. J Thromb Haemost. 2016;14(10):1908–16. https://doi.org/10.1111/jth.13441.
van den Biggelaar M, Hernandez-Fernaud JR, van den Eshof BL, Neilson LJ, Meijer AB, Mertens K, et al. Quantitative phosphoproteomics unveils temporal dynamics of thrombin signaling in human endothelial cells. Blood. 2014;123(12):e22–36. https://doi.org/10.1182/blood-2013-12-546036.
Striggow F, Riek-Burchardt M, Kiesel A, Schmidt W, Henrich-Noack P, Breder J, et al. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14(4):595–608. https://doi.org/10.1046/j.0953-816x.2001.01676.x.
Suo Z, Wu M, Ameenuddin S, Anderson HE, Zoloty JE, Citron BA, et al. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J Neurochem. 2002;80(4):655–66. https://doi.org/10.1046/j.0022-3042.2001.00745.x.
Wang H, Ubl JJ, Stricker R, Reiser G. Thrombin (PAR-1)-induced proliferation in astrocytes via MAPK involves multiple signaling pathways. Am J Physiol Cell Physiol. 2002;283(5):C1351–64. https://doi.org/10.1152/ajpcell.00001.2002.
Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21(10):1359–69. https://doi.org/10.1038/s41593-018-0242-x.
Luo W, Wang Y, Reiser G. Protease-activated receptors in the brain: receptor expression, activation, and functions in neurodegeneration and neuroprotection. Brain Res Rev. 2007;56(2):331–45. https://doi.org/10.1016/j.brainresrev.2007.08.002.
Wang Y, Luo W, Stricker R, Reiser G. Protease-activated receptor-1 protects rat astrocytes from apoptotic cell death via JNK-mediated release of the chemokine GRO/CINC-1. J Neurochem. 2006;98(4):1046–60. https://doi.org/10.1111/j.1471-4159.2006.03950.x.
Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J Neurosci. 1997;17(14):5316–26.
Nicole O, Goldshmidt A, Hamill CE, Sorensen SD, Sastre A, Lyuboslavsky P, et al. Activation of protease-activated receptor-1 triggers astrogliosis after brain injury. J Neurosci. 2005;25(17):4319–29. https://doi.org/10.1523/JNEUROSCI.5200-04.2005.
Brailoiu E, Shipsky MM, Yan G, Abood ME, Brailoiu GC. Mechanisms of modulation of brain microvascular endothelial cells function by thrombin. Brain Res. 1657;2017:167–75. https://doi.org/10.1016/j.brainres.2016.12.011.
Puech C, Delavenne X, He Z, Forest V, Mismetti P, Perek N. Direct oral anticoagulants are associated with limited damage of endothelial cells of the blood-brain barrier mediated by the thrombin/PAR-1 pathway. Brain Res. 1719;2019:57–63. https://doi.org/10.1016/j.brainres.2019.05.024.
Amar AP, Sagare AP, Zhao Z, Wang Y, Nelson AR, Griffin JH, et al. Can adjunctive therapies augment the efficacy of endovascular thrombolysis? A potential role for activated protein C. Neuropharmacology. 2018;134(Pt B):293–301. https://doi.org/10.1016/j.neuropharm.2017.09.021.
Wang Y, Zhao Z, Chow N, Rajput PS, Griffin JH, Lyden PD, et al. Activated protein C analog protects from ischemic stroke and extends the therapeutic window of tissue-type plasminogen activator in aged female mice and hypertensive rats. Stroke. 2013;44(12):3529–36. https://doi.org/10.1161/STROKEAHA.113.003350.
Lyden P, Pryor KE, Coffey CS, Cudkowicz M, Conwit R, Jadhav A, et al. Final results of the RHAPSODY trial: a multi-center, phase 2 trial using a continual reassessment method to determine the safety and tolerability of 3K3A-APC, a recombinant variant of human activated protein C, in combination with tissue plasminogen activator, mechanical thrombectomy or both in moderate to severe acute ischemic stroke. Ann Neurol. 2019;85(1):125–36. https://doi.org/10.1002/ana.25383.
Bonaca MP, Scirica BM, Braunwald E, Wiviott SD, Goto S, Nilsen DW, et al. New ischemic stroke and outcomes with vorapaxar versus placebo: results from the TRA 2 degrees P-TIMI 50 trial. J Am Coll Cardiol. 2014;64(22):2318–26. https://doi.org/10.1016/j.jacc.2014.07.997.
Jiang Y, Wu J, Hua Y, Keep RF, Xiang J, Hoff JT, et al. Thrombin-receptor activation and thrombin-induced brain tolerance. J Cereb Blood Flow Metab. 2002;22(4):404–10. https://doi.org/10.1097/00004647-200204000-00004.
Pike CJ, Vaughan PJ, Cunningham DD, Cotman CW. Thrombin attenuates neuronal cell death and modulates astrocyte reactivity induced by beta-amyloid in vitro. J Neurochem. 1996;66(4):1374–82. https://doi.org/10.1046/j.1471-4159.1996.66041374.x.
Masada T, Xi G, Hua Y, Keep RF. The effects of thrombin preconditioning on focal cerebral ischemia in rats. Brain Res. 2000;867(1–2):173–9 S0006-8993(00)02302-7 [pii].
Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, et al. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: role of the p22(phox)-containing NADPH oxidase. Circ Res. 2001;89(1):47–54. https://doi.org/10.1161/hh1301.092678.
Jiang Y, Wu J, Keep RF, Hua Y, Hoff JT, Xi G. Hypoxia-inducible factor-1alpha accumulation in the brain after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2002;22(6):689–96. https://doi.org/10.1097/00004647-200206000-00007.
Hua Y, Keep RF, Hoff JT, Xi G. Thrombin preconditioning attenuates brain edema induced by erythrocytes and iron. J Cereb Blood Flow Metab. 2003;23(12):1448–54. https://doi.org/10.1097/01.WCB.0000090621.86921.D5.
Zhou HJ, Tang T, Cui HJ, Yang AL, Luo JK, Lin Y, et al. Thrombin-triggered angiogenesis in rat brains following experimental intracerebral hemorrhage. J Neurosurg. 2012;117(5):920–8. https://doi.org/10.3171/2012.8.JNS112152.
Hua Y, Xi G, Keep RF, Wu J, Jiang Y, Hoff JT. Plasminogen activator inhibitor-1 induction after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2002;22(1):55–61. https://doi.org/10.1097/00004647-200201000-00007.
Hoffmann MC, Nitsch C, Scotti AL, Reinhard E, Monard D. The prolonged presence of glia-derived nexin, an endogenous protease inhibitor, in the hippocampus after ischemia-induced delayed neuronal death. Neuroscience. 1992;49(2):397–408. https://doi.org/10.1016/0306-4522(92)90105-b.
Wang Y, Luo W, Reiser G. Activation of protease-activated receptors in astrocytes evokes a novel neuroprotective pathway through release of chemokines of the growth-regulated oncogene/cytokine-induced neutrophil chemoattractant family. Eur J Neurosci. 2007;26(11):3159–68. https://doi.org/10.1111/j.1460-9568.2007.05938.x.
Malik A, Kanneganti TD. Function and regulation of IL-1alpha in inflammatory diseases and cancer. Immunol Rev. 2018;281(1):124–37. https://doi.org/10.1111/imr.12615.
Conway EM. Thrombin: coagulation’s master regulator of innate immunity. J Thromb Haemost. 2019;17(11):1785–9. https://doi.org/10.1111/jth.14586.
Nawroth PP, Handley DA, Esmon CT, Stern DM. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986;83(10):3460–4. https://doi.org/10.1073/pnas.83.10.3460.
Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21(15):5528–34.
Thornton P, McColl BW, Greenhalgh A, Denes A, Allan SM, Rothwell NJ. Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood. 2010;115(17):3632–9. https://doi.org/10.1182/blood-2009-11-252643.
Weinstock DM, Chang P, Aronson DL, Kessler CM. Comparison of plasma prothrombin and factor VII and urine prothrombin F1 concentrations in patients on long-term warfarin therapy and those in the initial phase. Am J Hematol. 1998;57(3):193–9. https://doi.org/10.1002/(sici)1096-8652(199803)57:3<193::aid-ajh2>3.0.co;2-q.
Gong Y, Xi G, Hu H, Gu Y, Huang F, Keep RF, et al. Increase in brain thrombin activity after experimental intracerebral hemorrhage. Acta Neurochir Suppl. 2008;105:47–50. https://doi.org/10.1007/978-3-211-09469-3_10.
Festoff BW, Smirnova IV, Ma J, Citron BA. Thrombin, its receptor and protease nexin I, its potent serpin, in the nervous system. Semin Thromb Hemost. 1996;22(3):267–71. https://doi.org/10.1055/s-2007-999018.
Meh DA, Siebenlist KR, Mosesson MW. Identification and characterization of the thrombin binding sites on fibrin. J Biol Chem. 1996;271(38):23121–5. https://doi.org/10.1074/jbc.271.38.23121.
Wilkinson DA, Pandey AS, Thompson BG, Keep RF, Hua Y, Xi G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology. 2018;134(Pt B):240–8. https://doi.org/10.1016/j.neuropharm.2017.09.033.
Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48(4):875–82; discussion 82-3. https://doi.org/10.1097/00006123-200104000-00037.
Kitaoka T, Hua Y, Xi G, Hoff JT, Keep RF. Delayed argatroban treatment reduces edema in a rat model of intracerebral hemorrhage. Stroke. 2002;33(12):3012–8. https://doi.org/10.1161/01.str.0000037673.17260.1b.
Nagatsuna T, Nomura S, Suehiro E, Fujisawa H, Koizumi H, Suzuki M. Systemic administration of argatroban reduces secondary brain damage in a rat model of intracerebral hemorrhage: histopathological assessment. Cerebrovasc Dis. 2005;19(3):192–200. https://doi.org/10.1159/000083466.
Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33(10):2478–84. https://doi.org/10.1161/01.str.0000032302.91894.0f.
Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38(2 Suppl):759–62. https://doi.org/10.1161/01.STR.0000247868.97078.10.
Nakamura T, Xi G, Park JW, Hua Y, Hoff JT, Keep RF. Holo-transferrin and thrombin can interact to cause brain damage. Stroke. 2005;36(2):348–52. https://doi.org/10.1161/01.STR.0000153044.60858.1b.
Mao S, Xi G, Keep RF, Hua Y. Role of lipocalin-2 in thrombin-induced brain injury. Stroke. 2016;47(4):1078–84. https://doi.org/10.1161/STROKEAHA.115.012153.
Manning L, Hirakawa Y, Arima H, Wang X, Chalmers J, Wang J, et al. Blood pressure variability and outcome after acute intracerebral haemorrhage: a post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol. 2014;13(4):364–73. https://doi.org/10.1016/S1474-4422(14)70018-3.
Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5(1):53–63. https://doi.org/10.1016/S1474-4422(05)70283-0.
Hua Y, Wu J, Keep RF, Nakamura T, Hoff JT, Xi G. Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006;58(3):542–50; discussion -50. https://doi.org/10.1227/01.Neu.0000197333.55473.Ad.
Wu J, Yang S, Xi G, Song S, Fu G, Keep RF, et al. Microglial activation and brain injury after intracerebral hemorrhage. Acta Neurochir Suppl. 2008;105:59–65. https://doi.org/10.1007/978-3-211-09469-3_13.
Gong Y, Xi GH, Keep RF, Hoff JT, Hua Y. Complement inhibition attenuates brain edema and neurological deficits induced by thrombin. Acta Neurochir Suppl. 2005;95:389–92. https://doi.org/10.1007/3-211-32318-x_79.
Giulian D, Baker TJ. Peptides released by ameboid microglia regulate astroglial proliferation. J Cell Biol. 1985;101(6):2411–5. https://doi.org/10.1083/jcb.101.6.2411.
Patel HC, Boutin H, Allan SM. Interleukin-1 in the brain: mechanisms of action in acute neurodegeneration. Ann N Y Acad Sci. 2003;992:39–47. https://doi.org/10.1111/j.1749-6632.2003.tb03136.x.
Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23(12):618–25. https://doi.org/10.1016/s0166-2236(00)01661-1.
Masada T, Hua Y, Xi G, Yang GY, Hoff JT, Keep RF. Attenuation of intracerebral hemorrhage and thrombin-induced brain edema by overexpression of interleukin-1 receptor antagonist. J Neurosurg. 2001;95(4):680–6. https://doi.org/10.3171/jns.2001.95.4.0680.
Cheng Y, Xi G, Jin H, Keep RF, Feng J, Hua Y. Thrombin-induced cerebral hemorrhage: role of protease-activated receptor-1. Transl Stroke Res. 2014;5(4):472–5. https://doi.org/10.1007/s12975-013-0288-8.
Wan S, Cheng Y, Jin H, Guo D, Hua Y, Keep RF, et al. Microglia activation and polarization after intracerebral hemorrhage in mice: the role of protease-activated receptor-1. Transl Stroke Res. 2016;7(6):478–87. https://doi.org/10.1007/s12975-016-0472-8.
Gingrich MB, Junge CE, Lyuboslavsky P, Traynelis SF. Potentiation of NMDA receptor function by the serine protease thrombin. J Neurosci. 2000;20(12):4582–95.
Lauer A, Cianchetti FA, Van Cott EM, Schlunk F, Schulz E, Pfeilschifter W, et al. Anticoagulation with the oral direct thrombin inhibitor dabigatran does not enlarge hematoma volume in experimental intracerebral hemorrhage. Circulation. 2011;124(15):1654–62. https://doi.org/10.1161/CIRCULATIONAHA.111.035972.
Schlunk F, Bohm M, Boulouis G, Qin T, Arbel M, Tamim I, et al. Secondary bleeding during acute experimental intracerebral hemorrhage. Stroke. 2019;50(5):1210–5. https://doi.org/10.1161/STROKEAHA.118.021732.
Bao X, Ye F, Wan S, Hua Y, Keep R, Garton HJL, et al. Role of protease activated receptor-1 in thrombin preconditioning against thrombin-induced brain injury in mice. Conditioning medicine. 2019;2(5):185–91.
Yang S, Hua Y, Nakamura T, Keep RF, Xi G. Up-regulation of brain ceruloplasmin in thrombin preconditioning. Acta Neurochir Suppl. 2006;96:203–6.
Liu H, Hua Y, Keep RF, Xi G. Brain ceruloplasmin expression after experimental intracerebral hemorrhage and protection against iron-induced brain injury. Transl Stroke Res. 2019;10(1):112–9. https://doi.org/10.1007/s12975-018-0669-0.
Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92(4):463–77. https://doi.org/10.1016/j.pneurobio.2010.08.001.
Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44. https://doi.org/10.1016/j.pneurobio.2013.11.003.
Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res. 2012;3(Suppl 1):25–38. https://doi.org/10.1007/s12975-012-0182-9.
Garton T, Hua Y, Xiang J, Xi G, Keep RF. Challenges for intraventricular hemorrhage research and emerging therapeutic targets. Expert Opin Ther Targets. 2017;21(12):1111–22. https://doi.org/10.1080/14728222.2017.1397628.
Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. 2009;40(4):1533–8. https://doi.org/10.1161/STROKEAHA.108.535419.
Rosen DS, Macdonald RL, Huo D, Goldenberg FD, Novakovic RL, Frank JI, et al. Intraventricular hemorrhage from ruptured aneurysm: clinical characteristics, complications, and outcomes in a large, prospective, multicenter study population. J Neurosurg. 2007;107(2):261–5. https://doi.org/10.3171/JNS-07/08/0261.
Mayfrank L, Kissler J, Raoofi R, Delsing P, Weis J, Kuker W, et al. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28(1):141–8. https://doi.org/10.1161/01.str.28.1.141.
Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017;389(10069):603–11. https://doi.org/10.1016/S0140-6736(16)32410-2.
Mazzola CA, Choudhri AF, Auguste KI, Limbrick DD Jr, Rogido M, Mitchell L, et al. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: management of posthemorrhagic hydrocephalus in premature infants. J Neurosurg Pediatr. 2014;14(Suppl 1):8–23. https://doi.org/10.3171/2014.7.PEDS14322.
Neary E, McCallion N, Kevane B, Cotter M, Egan K, Regan I, et al. Coagulation indices in very preterm infants from cord blood and postnatal samples. J Thromb Haemost. 2015;13(11):2021–30. https://doi.org/10.1111/jth.13130.
Bruschettini M, Romantsik O, Zappettini S, Banzi R, Ramenghi LA, Calevo MG. Heparin for the prevention of intraventricular haemorrhage in preterm infants. The Cochrane database of systematic reviews. 2016;5:Cd011718. https://doi.org/10.1002/14651858.CD011718.pub2.
Bruschettini M, Romantsik O, Zappettini S, Banzi R, Ramenghi LA, Calevo MG. Antithrombin for the prevention of intraventricular hemorrhage in very preterm infants. The Cochrane database of systematic reviews. 2016;3:Cd011636. https://doi.org/10.1002/14651858.CD011636.pub2.
Gao F, Liu F, Chen Z, Hua Y, Keep RF, Xi G. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab. 2014;34(3):489–94. https://doi.org/10.1038/jcbfm.2013.225.
Liu DZ, Ander BP, Xu H, Shen Y, Kaur P, Deng W, et al. Blood-brain barrier breakdown and repair by Src after thrombin-induced injury. Ann Neurol. 2010;67(4):526–33. https://doi.org/10.1002/ana.21924.
Liu DZ, Waldau B, Ander BP, Zhan X, Stamova B, Jickling GC, et al. Inhibition of Src family kinases improves cognitive function after intraventricular hemorrhage or intraventricular thrombin. J Cereb Blood Flow Metab. 2017;37(7):2359–67. https://doi.org/10.1177/0271678X16666291.
Xue M, Balasubramaniam J, Parsons KA, McIntyre IW, Peeling J, Del Bigio MR. Does thrombin play a role in the pathogenesis of brain damage after periventricular hemorrhage? Brain Pathol. 2005;15(3):241–9. https://doi.org/10.1111/j.1750-3639.2005.tb00527.x.
Lekic T, Manaenko A, Rolland W, Krafft PR, Peters R, Hartman RE, et al. Rodent neonatal germinal matrix hemorrhage mimics the human brain injury, neurological consequences, and post-hemorrhagic hydrocephalus. Exp Neurol. 2012;236(1):69–78. https://doi.org/10.1016/j.expneurol.2012.04.003.
Lekic T, Krafft PR, Klebe D, Flores J, Rolland WB, Tang J, et al. PAR-1, -4, and the mTOR pathway following germinal matrix hemorrhage. Acta Neurochir Suppl. 2016;121:213–6. https://doi.org/10.1007/978-3-319-18497-5_38.
Klebe D, Flores JJ, McBride DW, Krafft PR, Rolland WB, Lekic T, et al. Dabigatran ameliorates post-haemorrhagic hydrocephalus development after germinal matrix haemorrhage in neonatal rat pups. J Cereb Blood Flow Metab. 2017;37(9):3135–49. https://doi.org/10.1177/0271678X16684355.
Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 2017;23(8):997–1003. https://doi.org/10.1038/nm.4361.
Gao F, Zheng M, Hua Y, Keep RF, Xi G. Acetazolamide attenuates thrombin-induced hydrocephalus. Acta Neurochir Suppl. 2016;121:373–7. https://doi.org/10.1007/978-3-319-18497-5_64.
Wan Y, Hua Y, Garton HJL, Novakovic N, Keep RF, Xi G. Activation of epiplexus macrophages in hydrocephalus caused by subarachnoid hemorrhage and thrombin. CNS neuroscience & therapeutics. 2019;25(10):1134–41. https://doi.org/10.1111/cns.13203.
Hao XD, Le CS, Zhang HM, Shang DS, Tong LS, Gao F. Thrombin disrupts vascular endothelial-cadherin and leads to hydrocephalus via protease-activated receptors-1 pathway. CNS neuroscience & therapeutics. 2019;25(10):1142–50. https://doi.org/10.1111/cns.13129.
Wan Y, Gao F, Ye F, Yang W, Hua Y, Keep RF, et al. Effects of aging on hydrocephalus after intraventricular hemorrhage. Fluids and barriers of the CNS. 2020;17(1):8. https://doi.org/10.1186/s12987-020-0169-y.
Suzuki M, Kudo A, Otawara Y, Hirashima Y, Takaku A, Ogawa A. Extrinsic pathway of blood coagulation and thrombin in the cerebrospinal fluid after subarachnoid hemorrhage. Neurosurgery. 1999;44(3):487–93; discussion 93-4. https://doi.org/10.1097/00006123-199903000-00029.
Ikeda K, Asakura H, Futami K, Yamashita J. Coagulative and fibrinolytic activation in cerebrospinal fluid and plasma after subarachnoid hemorrhage. Neurosurgery. 1997;41(2):344–9; discussion 9-50. https://doi.org/10.1097/00006123-199708000-00002.
Peterson EW, Cardoso ER. The blood-brain barrier following experimental subarachnoid hemorrhage. Part 1: response to insult caused by arterial hypertension. J Neurosurg. 1983;58(3):338–44. https://doi.org/10.3171/jns.1983.58.3.0338.
Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26(11):1341–53. https://doi.org/10.1038/sj.jcbfm.9600283.
Helbok R, Ko SB, Schmidt JM, Kurtz P, Fernandez L, Choi HA, et al. Global cerebral edema and brain metabolism after subarachnoid hemorrhage. Stroke. 2011;42(6):1534–9. https://doi.org/10.1161/STROKEAHA.110.604488.
Sugawara T, Jadhav V, Ayer R, Chen W, Suzuki H, Zhang JH. Thrombin inhibition by argatroban ameliorates early brain injury and improves neurological outcomes after experimental subarachnoid hemorrhage in rats. Stroke. 2009;40(4):1530–2. https://doi.org/10.1161/strokeaha.108.531699.
Yan J, Manaenko A, Chen S, Klebe D, Ma Q, Caner B, et al. Role of SCH79797 in maintaining vascular integrity in rat model of subarachnoid hemorrhage. Stroke. 2013;44(5):1410–7. https://doi.org/10.1161/STROKEAHA.113.678474.
Kolias AG, Sen J, Belli A. Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: putative mechanisms and novel approaches. J Neurosci Res. 2009;87(1):1–11. https://doi.org/10.1002/jnr.21823.
Danura H, Schatlo B, Marbacher S, Kerkeni H, Diepers M, Remonda L, et al. Acute angiographic vasospasm and the incidence of delayed cerebral vasospasm: preliminary results. Acta Neurochir Suppl. 2015;120:187–90. https://doi.org/10.1007/978-3-319-04981-6_32.
Hirano K, Hirano M. Current perspective on the role of the thrombin receptor in cerebral vasospasm after subarachnoid hemorrhage. J Pharmacol Sci. 2010;114(2):127–33. https://doi.org/10.1254/jphs.10r03cp.
Ross R, Masuda J, Raines EW, Gown AM, Katsuda S, Sasahara M, et al. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990;248(4958):1009–12. https://doi.org/10.1126/science.2343305.
Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–312. https://doi.org/10.1101/gad.1653708.
Suzuki S, Suzuki M, Iwabuchi T, Kamata Y. Role of multiple cerebral microthrombosis in symptomatic cerebral vasospasm: with a case report. Neurosurgery. 1983;13(2):199–203. https://doi.org/10.1227/00006123-198308000-00018.
Suzuki S, Kimura M, Souma M, Ohkima H, Shimizu T, Iwabuchi T. Cerebral microthrombosis in symptomatic cerebral vasospasm--a quantitative histological study in autopsy cases. Neurol Med Chir (Tokyo). 1990;30(5):309–16. https://doi.org/10.2176/nmc.30.309.
Friedrich B, Muller F, Feiler S, Scholler K, Plesnila N. Experimental subarachnoid hemorrhage causes early and long-lasting microarterial constriction and microthrombosis: an in-vivo microscopy study. J Cereb Blood Flow Metab. 2012;32(3):447–55. https://doi.org/10.1038/jcbfm.2011.154.
Guo D, Wilkinson DA, Thompson BG, Pandey AS, Keep RF, Xi G, et al. MRI characterization in the acute phase of experimental subarachnoid hemorrhage. Transl Stroke Res. 2017;8(3):234–43. https://doi.org/10.1007/s12975-016-0511-5.
Clarke JV, Suggs JM, Diwan D, Lee JV, Lipsey K, Vellimana AK, et al. Microvascular platelet aggregation and thrombosis after subarachnoid hemorrhage: a review and synthesis. J Cereb Blood Flow Metab. 2020:271678X20921974. https://doi.org/10.1177/0271678X20921974.
Sehba FA, Mostafa G, Friedrich V Jr, Bederson JB. Acute microvascular platelet aggregation after subarachnoid hemorrhage. J Neurosurg. 2005;102(6):1094–100. https://doi.org/10.3171/jns.2005.102.6.1094.
Mayer SA, Kreiter KT, Copeland D, Bernardini GL, Bates JE, Peery S, et al. Global and domain-specific cognitive impairment and outcome after subarachnoid hemorrhage. Neurology. 2002;59(11):1750–8. https://doi.org/10.1212/01.wnl.0000035748.91128.c2.
Brathwaite S, Macdonald RL. Current management of delayed cerebral ischemia: update from results of recent clinical trials. Transl Stroke Res. 2014;5(2):207–26. https://doi.org/10.1007/s12975-013-0316-8.
Dienel A, Ammassam Veettil R, Hong SH, Matsumura K, Kumar TP, Yan Y, et al. Microthrombi correlates with infarction and delayed neurological deficits after subarachnoid hemorrhage in mice. Stroke. 2020;51(7):2249–54. https://doi.org/10.1161/STROKEAHA.120.029753.
Dasenbrock HH, Angriman F, Smith TR, Gormley WB, Frerichs KU, Aziz-Sultan MA, et al. Readmission after aneurysmal subarachnoid hemorrhage: a Nationwide Readmission Database analysis. Stroke. 2017;48(9):2383–90. https://doi.org/10.1161/STROKEAHA.117.016702.
Adil SM, Liu B, Charalambous LT, Kiyani M, Gramer R, Swisher CB, et al. Healthcare economics of hydrocephalus after aneurysmal subarachnoid hemorrhage in the United States. Transl Stroke Res. 2019;10(6):650–63. https://doi.org/10.1007/s12975-019-00697-9.
Okubo S, Strahle J, Keep RF, Hua Y, Xi G. Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke. 2013;44(2):547–50. https://doi.org/10.1161/STROKEAHA.112.662312.
Golanov EV, Bovshik EI, Wong KK, Pautler RG, Foster CH, Federley RG, et al. Subarachnoid hemorrhage - induced block of cerebrospinal fluid flow: role of brain coagulation factor III (tissue factor). J Cereb Blood Flow Metab. 2018;38(5):793–808. https://doi.org/10.1177/0271678X17701157.
Thomas AJ, Ogilvy CS, Griessenauer CJ, Hanafy KA. Macrophage CD163 expression in cerebrospinal fluid: association with subarachnoid hemorrhage outcome. J Neurosurg. 2018;131(1):47–53. https://doi.org/10.3171/2018.2.JNS172828.
Mohme M, Sauvigny T, Mader MM, Schweingruber N, Maire CL, Runger A, et al. Immune characterization in aneurysmal subarachnoid hemorrhage reveals distinct monocytic activation and chemokine patterns. Transl Stroke Res. 2019. https://doi.org/10.1007/s12975-019-00764-1.
Tsurutani H, Ohkuma H, Suzuki S. Effects of thrombin inhibitor on thrombin-related signal transduction and cerebral vasospasm in the rabbit subarachnoid hemorrhage model. Stroke. 2003;34(6):1497–500. https://doi.org/10.1161/01.Str.0000070424.38138.30.
Kameda K, Kikkawa Y, Hirano M, Matsuo S, Sasaki T, Hirano K. Combined argatroban and anti-oxidative agents prevents increased vascular contractility to thrombin and other ligands after subarachnoid haemorrhage. Br J Pharmacol. 2012;165(1):106–19. https://doi.org/10.1111/j.1476-5381.2011.01485.x.
Zhang Z, Nagata I, Kikuchi H, Xue JH, Sakai N, Sakai H, et al. Broad-spectrum and selective serine protease inhibitors prevent expression of platelet-derived growth factor-BB and cerebral vasospasm after subarachnoid hemorrhage: vasospasm caused by cisternal injection of recombinant platelet-derived growth factor-BB. Stroke. 2001;32(7):1665–72. https://doi.org/10.1161/01.str.32.7.1665.
Simard JM, Tosun C, Ivanova S, Kurland DB, Hong C, Radecki L, et al. Heparin reduces neuroinflammation and transsynaptic neuronal apoptosis in a model of subarachnoid hemorrhage. Transl Stroke Res. 2012;3(Suppl 1):155–65. https://doi.org/10.1007/s12975-012-0166-9.
Simard JM, Aldrich EF, Schreibman D, James RF, Polifka A, Beaty N. Low-dose intravenous heparin infusion in patients with aneurysmal subarachnoid hemorrhage: a preliminary assessment. J Neurosurg. 2013;119(6):1611–9. https://doi.org/10.3171/2013.8.JNS1337.
James RF, Khattar NK, Aljuboori ZS, Page PS, Shao EY, Carter LM, et al. Continuous infusion of low-dose unfractionated heparin after aneurysmal subarachnoid hemorrhage: a preliminary study of cognitive outcomes. J Neurosurg. 2018:1–8. https://doi.org/10.3171/2017.11.JNS17894.
Siironen J, Juvela S, Varis J, Porras M, Poussa K, Ilveskero S, et al. No effect of enoxaparin on outcome of aneurysmal subarachnoid hemorrhage: a randomized, double-blind, placebo-controlled clinical trial. J Neurosurg. 2003;99(6):953–9. https://doi.org/10.3171/jns.2003.99.6.0953.
Wurm G, Tomancok B, Nussbaumer K, Adelwohrer C, Holl K. Reduction of ischemic sequelae following spontaneous subarachnoid hemorrhage: a double-blind, randomized comparison of enoxaparin versus placebo. Clin Neurol Neurosurg. 2004;106(2):97–103. https://doi.org/10.1016/j.clineuro.2004.01.006.
Lindbohm JV, Kaprio J, Jousilahti P, Salomaa V, Korja M. Sex, smoking, and risk for subarachnoid hemorrhage. Stroke. 2016;47(8):1975–81. https://doi.org/10.1161/STROKEAHA.116.012957.
Sundstrom J, Soderholm M, Soderberg S, Alfredsson L, Andersson M, Bellocco R, et al. Risk factors for subarachnoid haemorrhage: a nationwide cohort of 950 000 adults. Int J Epidemiol. 2019;48(6):2018–25. https://doi.org/10.1093/ije/dyz163.
Turan N, Heider RA, Zaharieva D, Ahmad FU, Barrow DL, Pradilla G. Sex differences in the formation of intracranial aneurysms and incidence and outcome of subarachnoid hemorrhage: review of experimental and human studies. Transl Stroke Res. 2016;7(1):12–9. https://doi.org/10.1007/s12975-015-0434-6.
Whiteley WN, Adams HP Jr, Bath PM, Berge E, Sandset PM, Dennis M, et al. Targeted use of heparin, heparinoids, or low-molecular-weight heparin to improve outcome after acute ischaemic stroke: an individual patient data meta-analysis of randomised controlled trials. Lancet Neurol. 2013;12(6):539–45. https://doi.org/10.1016/S1474-4422(13)70079-6.
Sandercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. The Cochrane database of systematic reviews. 2015;3:CD000024. https://doi.org/10.1002/14651858.CD000024.pub4.
Lansberg MG, O'Donnell MJ, Khatri P, Lang ES, Nguyen-Huynh MN, Schwartz NE, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e601S–e36S. https://doi.org/10.1378/chest.11-2302.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–418. https://doi.org/10.1161/STR.0000000000000211.
Barreto AD, Alexandrov AV, Lyden P, Lee J, Martin-Schild S, Shen L, et al. The argatroban and tissue-type plasminogen activator stroke study: final results of a pilot safety study. Stroke. 2012;43(3):770–5. https://doi.org/10.1161/STROKEAHA.111.625574.
Barreto AD, Ford GA, Shen L, Pedroza C, Tyson J, Cai C, et al. Randomized, multicenter trial of ARTSS-2 (argatroban with recombinant tissue plasminogen activator for acute stroke). Stroke. 2017;48(6):1608–16. https://doi.org/10.1161/STROKEAHA.117.016720.
Butcher KS, Ng K, Sheridan P, Field TS, Coutts SB, Siddiqui M, et al. Dabigatran treatment of acute noncardioembolic ischemic stroke. Stroke. 2020;51(4):1190–8. https://doi.org/10.1161/STROKEAHA.119.027569.
Kate M, Gioia L, Buck B, Sivakumar L, Jeerakathil T, Shuaib A, et al. Dabigatran therapy in acute ischemic stroke patients without atrial fibrillation. Stroke. 2015;46(9):2685–7. https://doi.org/10.1161/STROKEAHA.115.010383.
Chen B, Friedman B, Whitney MA, Winkle JA, Lei IF, Olson ES, et al. Thrombin activity associated with neuronal damage during acute focal ischemia. J Neurosci. 2012;32(22):7622–31. https://doi.org/10.1523/JNEUROSCI.0369-12.2012.
Bushi D, Chapman J, Katzav A, Shavit-Stein E, Molshatzki N, Maggio N, et al. Quantitative detection of thrombin activity in an ischemic stroke model. Journal of molecular neuroscience : MN. 2013;51(3):844–50. https://doi.org/10.1007/s12031-013-0072-y.
Machida T, Dohgu S, Takata F, Matsumoto J, Kimura I, Koga M, et al. Role of thrombin-PAR1-PKCtheta/delta axis in brain pericytes in thrombin-induced MMP-9 production and blood-brain barrier dysfunction in vitro. Neuroscience. 2017;350:146–57. https://doi.org/10.1016/j.neuroscience.2017.03.026.
Gavard J, Gutkind JS. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Galpha12/13 and Galpha11/q. J Biol Chem. 2008;283(44):29888–96. https://doi.org/10.1074/jbc.M803880200.
Stein ES, Itsekson-Hayosh Z, Aronovich A, Reisner Y, Bushi D, Pick CG, et al. Thrombin induces ischemic LTP (iLTP): implications for synaptic plasticity in the acute phase of ischemic stroke. Sci Rep. 2015;5:7912. https://doi.org/10.1038/srep07912.
Fujimoto S, Katsuki H, Ohnishi M, Takagi M, Kume T, Akaike A. Thrombin induces striatal neurotoxicity depending on mitogen-activated protein kinase pathways in vivo. Neuroscience. 2007;144(2):694–701. https://doi.org/10.1016/j.neuroscience.2006.09.049.
Rajput PS, Lyden PD, Chen B, Lamb JA, Pereira B, Lamb A, et al. Protease activated receptor-1 mediates cytotoxicity during ischemia using in vivo and in vitro models. Neuroscience. 2014;281:229–40. https://doi.org/10.1016/j.neuroscience.2014.09.038.
Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, et al. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proc Natl Acad Sci U S A. 2003;100(22):13019–24. https://doi.org/10.1073/pnas.2235594100.
Wang J, Jin H, Hua Y, Keep RF, Xi G. Role of protease-activated receptor-1 in brain injury after experimental global cerebral ischemia. Stroke. 2012;43(9):2476–82. https://doi.org/10.1161/strokeaha.112.661819.
Morris DC, Zhang L, Zhang ZG, Lu M, Berens KL, Brown PM, et al. Extension of the therapeutic window for recombinant tissue plasminogen activator with argatroban in a rat model of embolic stroke. Stroke. 2001;32(11):2635–40. https://doi.org/10.1161/hs1101.097390.
Lyden P, Pereira B, Chen B, Zhao L, Lamb J, Lei IF, et al. Direct thrombin inhibitor argatroban reduces stroke damage in 2 different models. Stroke. 2014;45(3):896–9. https://doi.org/10.1161/STROKEAHA.113.004488.
Ohyama H, Hosomi N, Takahashi T, Mizushige K, Kohno M. Thrombin inhibition attenuates neurodegeneration and cerebral edema formation following transient forebrain ischemia. Brain Res. 2001;902(2):264–71. https://doi.org/10.1016/s0006-8993(01)02354-x.
Hawkins BT, Gu YH, Izawa Y, del Zoppo GJ. Dabigatran abrogates brain endothelial cell permeability in response to thrombin. J Cereb Blood Flow Metab. 2015;35(6):985–92. https://doi.org/10.1038/jcbfm.2015.9.
Choi HJ, Kim NE, Kim J, An S, Yang SH, Ha J, et al. Dabigatran reduces endothelial permeability through inhibition of thrombin-induced cytoskeleton reorganization. Thromb Res. 2018;167:165–71. https://doi.org/10.1016/j.thromres.2018.04.019.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. https://doi.org/10.1056/NEJMoa0905561.
Sinha RK, Wang Y, Zhao Z, Xu X, Burnier L, Gupta N, et al. PAR1 biased signaling is required for activated protein C in vivo benefits in sepsis and stroke. Blood. 2018;131(11):1163–71. https://doi.org/10.1182/blood-2017-10-810895.
Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C, protease activated receptor 1, and neuroprotection. Blood. 2018;132(2):159–69. https://doi.org/10.1182/blood-2018-02-769026.
Gall SL, Donnan G, Dewey HM, Macdonell R, Sturm J, Gilligan A, et al. Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology. 2010;74(12):975–81. https://doi.org/10.1212/WNL.0b013e3181d5a48f.
Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915–26. https://doi.org/10.1016/S1474-4422(08)70193-5.
Funding
YH, RFK, and GX are supported by grants NS-096917, NS-106746, NS-112394, and NS116786 from the National Institutes of Health (NIH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ye, F., Garton, H.J.L., Hua, Y. et al. The Role of Thrombin in Brain Injury After Hemorrhagic and Ischemic Stroke. Transl. Stroke Res. 12, 496–511 (2021). https://doi.org/10.1007/s12975-020-00855-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-020-00855-4