Abstract
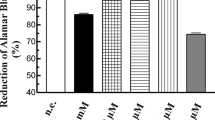
In this study, the effects of pharmacological concentrations of melatonin (1 μM–1 mM) on human pancreatic stellate cells (HPSCs) have been examined. Cell type–specific markers and expression of melatonin receptors were analyzed by western blot analysis. Changes in intracellular free Ca2+ concentration were followed by fluorimetric analysis of fura-2–loaded cells. Reduced glutathione (GSH) and oxidized glutathione (GSSG) levels were determined by fluorescence techniques. Production of reactive oxygen species (ROS) was monitored following 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester and MitoSOX™ Red–derived fluorescence. Cell viability was studied using the AlamarBlue® test. Cultured cells expressed markers typical of stellate cells. However, cell membrane receptors for melatonin could not be detected. Thapsigargin, bradykinin, or melatonin induced changes in intracellular free Ca2+ concentration. In the presence of the indole, a decrease in the GSH/GSSG ratio was observed that depended on the concentration of melatonin used. Furthermore, the indole evoked a concentration-dependent increase in ROS production in the mitochondria and in the cytosol. Finally, melatonin decreased HPSC viability in a time and concentration-dependent manner. We conclude that melatonin, at pharmacological concentrations, induces changes in the oxidative state of HPSC. This might regulate cellular viability and could not involve specific plasma membrane receptors.







Similar content being viewed by others
Abbreviations
- [Ca2+]c :
-
Intracellular free Ca2+ concentration
- CM-H2DCFDA:
-
5-(and-6)-Chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester
- ER:
-
Endoplasmic reticulum
- Fura-2-AM:
-
Fura-2-acetoxymethyl ester
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- HBSS:
-
Hank’s balanced salts
- HPSCs:
-
Human pancreatic stellate cells
- H2O2 :
-
Hydrogen peroxide
- ROS:
-
Reactive oxygen species
- RPSCs:
-
Rat pancreatic stellate cells
- SERCA:
-
Sarcoendoplasmic reticulum Ca2+-ATPase
- Tps:
-
Thapsigargin
- α-sma:
-
Alpha-smooth muscle actin
References
Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 71:2997–3025. https://doi.org/10.1007/s00018-014-1579-2
Angelova PR, Abramov AY (2018) Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett 592:692–702. https://doi.org/10.1002/1873-3468.12964
Apte M (2011) Isolation of quiescent pancreatic stellate cells from rat and human pancreas. Pancreapedia: Exocrine Pancreas Knowledge Base 10:434–443. https://doi.org/10.3998/panc.2011.10
Bonnefont-Rousselot D, Collin F (2010) Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicology 278:55–67. https://doi.org/10.1016/j.tox.2010.04.008
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Chetboun M, Abitbol G, Rozenberg K, Rozenfeld H, Deutsch A, Sampson SR, Rosenzweig T (2012) Maintenance of redox state and pancreatic beta-cell function: role of leptin and adiponectin. J Cell Biochem 113:1966–1976. https://doi.org/10.1002/jcb.24065
Chovancova B, Hudecova S, Lencesova L, Babula P, Rezuchova I, Penesova A, Grman M, Moravcik R, Zeman M, Krizanova O (2017) Melatonin-induced changes in cytosolic calcium might be responsible for apoptosis induction in tumour cells. Cell Physiol Biochem 44:763–777. https://doi.org/10.1159/000485290
Del Castillo-Vaquero A, Salido GM, González A (2010) Melatonin induces calcium release from CCK-8- and thapsigargin-sensitive cytosolic stores in pancreatic AR42J cells. J Pineal Res 49:256–263. https://doi.org/10.1111/j.1600-079X.2010.00790.x
García-Giménez JL, Romá-Mateo C, Pérez-Machado G, Peiró-Chova L, Pallardó FV (2017) Role of glutathione in the regulation of epigenetic mechanisms in disease. Free Radic Biol Med 112:36–48. https://doi.org/10.1016/j.freeradbiomed.2017.07.008
García-Marín R, de Miguel M, Fernández-Santos JM, Carrillo-Vico A, Utrilla JC, Morillo-Bernal J, Díaz-Parrado E, Rodríguez-Prieto I, Guerrero JM, Martín-Lacave I (2012) Melatonin-synthesizing enzymes and melatonin receptor in rat thyroid cells. Histol Histopathol 27:1429–1438. https://doi.org/10.14670/HH-27.1429
González A, del Castillo-Vaquero A, Miró-Morán A, Tapia JA, Salido GM (2011a) Melatonin reduces pancreatic tumor cell viability by altering mitochondrial physiology. J Pineal Res 50:250–260. https://doi.org/10.1111/j.1600-079X.2010.00834.x
Gonzalez A, Salido GM (2016) Determination of reactive oxygen species production in pancreatic acinar cells. Pancreapedia: Exocrine Pancreas Knowledge Base. doi: https://doi.org/10.3998/panc.2016.32
Gonzalez A, Santofimia-Castaño P, Salido GM (2011b). Culture of pancreatic AR42J cell for use as a model for acinar cell function. Pancreapedia: Exocrine Pancreas Knowledge Base doi: https://doi.org/10.3998/panc.2011.26
Gryshchenko O, Gerasimenko JV, Gerasimenko OV, Petersen OH (2016) Ca(2+) signals mediated by bradykinin type 2 receptors in normal pancreatic stellate cells can be inhibited by specific Ca(2+) channel blockade. J Physiol 594:281–293. https://doi.org/10.1113/JP271468
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Jaworek J, Leja-Szpak A, Bonior J, Nawrot K, Tomaszewska R, Stachura J, Sendur R, Pawlik W, Brzozowski T, Konturek SJ (2003) Protective effect of melatonin and its precursor L-tryptophan on acute pancreatitis induced by caerulein overstimulation or ischemia/reperfusion. J Pineal Res 34:40–52
Jaworek J, Nawrot K, Konturek SJ, Leja-Szpak A, Thor P, Pawlik WW (2004) Melatonin and its precursor, L-tryptophan: influence on pancreatic amylase secretion in vivo and in vitro. J Pineal Res 36:155–164
Leja-Szpak A, Jaworek J, Pierzchalski P, Reiter RJ (2010) Melatonin induces pro-apoptotic signaling pathway in human pancreatic carcinoma cells (PANC-1). J Pineal Res 49:248–255. https://doi.org/10.1111/j.1600-079X.2010.00789.x
Limón-Pacheco JH, Gonsebatt ME (2010) The glutathione system and its regulation by neurohormone melatonin in the central nervous system. Cent Nerv Syst Agents Med Chem 10:287–297
Mahadevan D, Von Hoff DD (2007) Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther 6:1186–1197
McCarroll JA, Naim S, Sharbeen G, Russia N, Lee J, Kavallaris M, Goldstein D, Phillips PA (2014) Role of pancreatic stellate cells in chemoresistance in pancreatic cancer. Front Physiol 9(5):141
Muñoz-Casares FC, Padillo FJ, Briceño J, Collado JA, Muñoz-Castañeda JR, Ortega R, Cruz A, Túnez I, Montilla P, Pera C, Muntané J (2006) Melatonin reduces apoptosis and necrosis induced by ischemia/reperfusion injury of the pancreas. J Pineal Res 40:195–203. https://doi.org/10.1111/j.1600-079X.2005.00291.x
Nath R, Raser KJ, Hajimohammadreza I, Wang KK (1997) Thapsigargin induces apoptosis in SH-SY5Y neuroblastoma cells and cerebrocortical cultures. Biochem Mol Biol Int 43:197–205
Nielsen SF, Thastrup O, Pedersen R, Olsen CE, Christensen SB (1995) Structure-activity relationships of analogues of thapsigargin modified at O-11 and O-12. J Med Chem 38:272–276
Pothula SP, Xu Z, Goldstein D, Pirola RC, Wilson JS, Apte MV (2016) Key role of pancreatic stellate cells in pancreatic cancer. Cancer Lett 381:194–200. https://doi.org/10.1016/j.canlet.2015.10.035
Sánchez-Sánchez AM, Martín V, García-Santos G, Rodríguez-Blanco J, Casado-Zapico S, Suarez-Garnacho S, Antolín I, Rodriguez C (2011) Intracellular redox state as determinant for melatonin antiproliferative vs cytotoxic effects in cancer cells. Free Radic Res 45:1333–1341. https://doi.org/10.3109/10715762.2011.623700
Sallinen P, Saarela S, Ilves M, Vakkuri O, Leppäluoto J (2005) The expression of MT1 and MT2 melatonin receptor mRNA in several rat tissues. Life Sci 76:1123–1134
Santofimia-Castaño P, Clea Ruy D, Garcia-Sanchez L, Jimenez-Blasco D, Fernandez-Bermejo M, Bolaños JP, Salido GM, Gonzalez A (2015a) Melatonin induces the expression of Nrf2-regulated antioxidant enzymes via PKC and Ca2+ influx activation in mouse pancreatic acinar cells. Free Radic Biol Med 87:226–236. https://doi.org/10.1016/j.freeradbiomed.2015.06.033.
Santofimia-Castaño P, Garcia-Sanchez L, Ruy DC, Sanchez-Correa B, Fernandez-Bermejo M, Tarazona R, Salido GM, Gonzalez A (2015b) Melatonin induces calcium mobilization and influences cell proliferation independently of MT1/MT2 receptor activation in rat pancreatic stellate cells. Cell Biol Toxicol 31:95–110. https://doi.org/10.1007/s10565-015-9297-6.
Santofimia-Castaño P, Ruy DC, Salido GM, González A (2013a) Melatonin modulates Ca2+ mobilization and amylase release in response to cholecystokinin octapeptide in mouse pancreatic acinar cells. J Physiol Biochem 69:897–908. https://doi.org/10.1007/s13105-013-0267-2
Santofimia-Castaño P, Salido GM, González A (2013b) Ebselen alters mitochondrial physiology and reduces viability of rat hippocampal astrocytes. DNA Cell Biol 32:147–155. https://doi.org/10.1089/dna.2012.1939
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT (2012) Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 351:152–166
Strobel O, Dadabaeva N, Felix K, Hackert T, Giese NA, Jesenofsky R, Werner J (2016) Isolation and culture of primary human pancreatic stellate cells that reflect the context of their tissue of origin. Langenbeck's Arch Surg 401:89–97. https://doi.org/10.1007/s00423-015-1343-6
Sutton R, Petersen OH, Pandol SJ (2008) Pancreatitis and calcium signalling: report of an international workshop. Pancreas 36:e1–e14. https://doi.org/10.1097/MPA.0b013e3181675010
Zha M, Li F, Xu W, Chen B, Sun Z (2014) Isolation and characterization of islet stellate cells in rat. Islets 6:e28701. https://doi.org/10.4161/isl.28701
Funding
This study was partly funded by the Ministerio de Economía y Competitividad (BFU2016-79259-R; UNEX13-1E-1608) and Junta de Extremadura-FEDER (IB16006). The funding sources had no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Estaras, M., Moreno, N., Santofimia-Castaño, P. et al. Melatonin induces reactive oxygen species generation and changes in glutathione levels and reduces viability in human pancreatic stellate cells. J Physiol Biochem 75, 185–197 (2019). https://doi.org/10.1007/s13105-019-00671-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-019-00671-x