Abstract
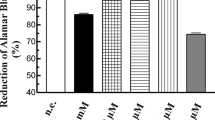
In this study, the effects of melatonin (1 μM–1 mM) on pancreatic stellate cells (PSC) have been examined. Cell viability and proliferation, caspase-3 activation, and the expression of cyclin A and cyclin D were analyzed. Our results show that melatonin decreased PSC viability in a time- and concentration-dependent manner. This effect was not inhibited by treatment of cells with MT1, MT2, calmodulin, or ROR-alpha inhibitors prior to melatonin addition. Activation of caspase-3 in response to melatonin was detected. The expression of cyclin A and cyclin D was decreased in cells treated with melatonin. Finally, changes in BrdU incorporation into the newly synthesized DNA of proliferating cells were also observed in the presence of melatonin. We conclude that melatonin, at pharmacological concentrations, modulates proliferation of PSC through activation of apoptosis and involving crucial regulators of the cell cycle. These actions might not require specific melatonin receptors. Our observations suggest that melatonin, at high doses, could potentially exert anti-fibrotic effects and, thus, could be taken into consideration as supportive treatment in the therapy of pancreatic diseases.




Similar content being viewed by others
Abbreviations
- BrdU:
-
5-bromo-2-deoxyuridine
- FSC:
-
Forward scatter
- KN93:
-
N-[2-[[[3-(4-Chlorophenyl)-2-propenyl]methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulphonamide
- PSCs:
-
Pancreatic stellate cells
- SCC:
-
Side scatter
- SR1001:
-
N-[4-Methyl-5-[[[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]amino]sulfonyl]-2-thiazolylacetamide
- Tps:
-
Thapsigargin
References
Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 71:2997–3025. https://doi.org/10.1007/s00018-014-1579-2
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Chen CQ, Fichna J, Bashashati M, Li YY, Storr M (2011) Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol 17:3888–3898. https://doi.org/10.3748/wjg.v17.i34.3888
Chidawanyika T, Sergison E, Cole M, Mark K, Supattapone S (2018) SEC24A identified as an essential mediator of thapsigargin-induced cell death in a genome-wide CRISPR/Cas9 screen. Cell Death Discov 4:115. https://doi.org/10.1038/s41420-018-0135-5
Del Castillo-Vaquero A, Salido GM, González A (2010) Melatonin induces calcium release from CCK-8- and thapsigargin-sensitive cytosolic stores in pancreatic AR42J cells. J Pineal Res 49:256–263. https://doi.org/10.1111/j.1600-079X.2010.00790.x
El-Magd MA, Mohamed Y, El-Shetry ES, Elsayed SA, Abo Gazia M, Abdel-Aleem GA, Shafik NM, Abdo WS, El-Desouki NI, Basyony MA (2019) Melatonin maximizes the therapeutic potential of non-preconditioned MSCs in a DEN-induced rat model of HCC. Biomed Pharmacother 114:108732. https://doi.org/10.1016/j.biopha.2019.108732
Estaras M, Moreno N, Santofimia-Castaño P, Martinez-Morcillo S, Roncero V, Blanco G, Lopez D, Fernandez-Bermejo M, Mateos JM, Iovanna JL, Salido GM, Gonzalez A (2019) Melatonin induces reactive oxygen species generation and changes in glutathione levels and reduces viability in human pancreatic stellate cells. J Physiol Biochem 75:185–197. https://doi.org/10.1007/s13105-019-00671-x
Ferdek PE, Jakubowska MA (2017) Biology of pancreatic stellate cells—more than just pancreatic cancer. Pflugers Arch 469:1039–1050. https://doi.org/10.1007/s00424-017-1968-0
García-Marín R, de Miguel M, Fernández-Santos JM, Carrillo-Vico A, Utrilla JC, Morillo-Bernal J, Díaz-Parrado E, Rodríguez-Prieto I, Guerrero JM, Martín-Lacave I (2012) Melatonin-synthesizing enzymes and melatonin receptor in rat thyroid cells. Histol Histopathol 27:1429–1438. https://doi.org/10.14670/HH-27.1429
González A, del Castillo-Vaquero A, Miró-Morán A, Tapia JA, Salido GM (2011) Melatonin reduces pancreatic tumor cell viability by altering mitochondrial physiology. J Pineal Res 50:250–260. https://doi.org/10.1111/j.1600-079X.2010.00834.x
Gryshchenko O, Gerasimenko JV, Gerasimenko OV, Petersen OH (2016) Ca2+ signals mediated by bradykinin type 2 receptors in normal pancreatic stellate cells can be inhibited by specific Ca2+ channel blockade. J Physiol 594:281–293. https://doi.org/10.1113/JP271468
Han J, Xu Y, Yu CX, Shen J, Wei YM (2008) Melatonin reverses the expression of morphine-induced conditioned place preference through its receptors within central nervous system in mice. Eur J Pharmacol 594:125–131. https://doi.org/10.1016/j.ejphar.2008.07.049
Hardeland R (2009) Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors 35:183–192. https://doi.org/10.1002/biof.23
Hector A, McAnulty C, Piché-Lemieux MÉ, Alves-Pires C, Buée-Scherrer V, Buée L, Brouillette J (2020) Tau hyperphosphorylation induced by the anesthetic agent ketamine/xylazine involved the calmodulin-dependent protein kinase II. FASEB J 34:2968–2977. https://doi.org/10.1096/fj.201902135R
Heo JS, Pyo S, Lim JY, Yoon DW, Kim BY, Kim JH, Kim GJ, Lee SG, Kim J (2019) Biological effects of melatonin on human adipose-derived mesenchymal stem cells. Int J Mol Med 44:2234–2244. https://doi.org/10.3892/ijmm.2019.4356
Jaworek J, Leja-Szpak A (2014) Melatonin influences pancreatic cancerogenesis. Histol Histopathol 29:423–431. https://doi.org/10.14670/HH-29.10.423
Jaworek J, Leja-Szpak A, Bonior J, Nawrot K, Tomaszewska R, Stachura J, Sendur R, Pawlik W, Brzozowski T, Konturek SJ (2003) Protective effect of melatonin and its precursor L-tryptophan on acute pancreatitis induced by caerulein overstimulation or ischemia/reperfusion. J Pineal Res 34:40–52
Jaworek J, Nawrot K, Konturek SJ, Leja-Szpak A, Thor P, Pawlik WW (2004) Melatonin and its precursor, L-tryptophan: influence on pancreatic amylase secretion in vivo and in vitro. J Pineal Res 36:155–164
Leja-Szpak A, Jaworek J, Pierzchalski P, Reiter RJ (2010) Melatonin induces pro-apoptotic signaling pathway in human pancreatic carcinoma cells (PANC-1). J Pineal Res 49:248–255. https://doi.org/10.1111/j.1600-079X.2010.00789.x
Li Y, Feng C, Gao M, Jin M, Liu T, Yuan Y, Yan G, Gong R, Sun Y, He M, Fu Y, Zhang L, Huang Q, Ding F, Ma W, Bi Z, Xu C, Sukhareva N, Bamba D, Reiters R, Yang F, Cai B, Yang L (2019) MicroRNA-92b-5p modulates melatonin-mediated osteogenic differentiation of bone marrow mesenchymal stem cells by targeting ICAM-1. J Cell Mol Med 23:6140–6153. https://doi.org/10.1111/jcmm.14490
Li J, Zhao YR, Tian Z (2019) Roles of hepatic stellate cells in acute liver failure: from the perspective of inflammation and fibrosis. World J Hepatol 11:412–420. https://doi.org/10.4254/wjh.v11.i5.412
Liu L, Zhu Y, Xu Y, Reiter RJ (2011) Melatonin delays cell proliferation by inducing G1 and G2/M phase arrest in a human osteoblastic cell line hFOB 1.19. J Pineal Res 50:222–231. https://doi.org/10.1111/j.1600-079X.2010.00832.x
Liu L, Xu Y, Reiter RJ (2013) Melatonin inhibits the proliferation of human osteosarcoma cell line MG-63. Bone 55:432–438. https://doi.org/10.1016/j.bone.2013.02.021
Loukil A, Cheung CT, Bendris N, Lemmers B, Peter M, Blanchard JM (2015) Cyclin A2: at the crossroads of cell cycle and cell invasion. World J Biol Chem 6:346–650. https://doi.org/10.4331/wjbc.v6.i4.346
Mahadevan D, Von Hoff DD (2007) Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther 6:1186–1197
Martín-Muñoz P, Ortega Ferrusola C, Vizuete G, Plaza Davila M, Rodriguez Martínez H, Peña FJ (2015) Depletion of intracellular thiols and increased production of 4-hydroxynonenal that occur during cryopreservation of stallion spermatozoa lead to caspase activation loss of motility and cell death. Biol Reprod 93:143. https://doi.org/10.1095/biolreprod.115.132878
McCarroll JA, Naim S, Sharbeen G, Russia N, Lee J, Kavallaris M, Goldstein D, Phillips PA (2014) Role of pancreatic stellate cells in chemoresistance in pancreatic cancer. Front Physiol 5:141. https://doi.org/10.3389/fphys.2014.00141
Modi Y, Shaaban H, Gauchan D, Maroules M, Parikh N, Guron G (2014) Primary clear cell ductal adenocarcinoma of the pancreas: a case report and clinicopathologic literature review. J Cancer Res Ther 10:773–776. https://doi.org/10.4103/0973-1482.136043
Muñoz-Casares FC, Padillo FJ, Briceño J, Collado JA, Muñoz-Castañeda JR, Ortega R, Cruz A, Túnez I, Montilla P, Pera C, Muntané J (2006) Melatonin reduces apoptosis and necrosis induced by ischemia/reperfusion injury of the pancreas. J Pineal Res 40:195–203. https://doi.org/10.1111/j.1600-079X.2005.00291.x
Ortega-Ferrusola C, Anel-López L, Martin Muñoz P, Ortiz-Rodriguez JM, Gil MC, Alvarez M, de Paz P, Ezquerra LJ, Masot AJ, Redondo E, Anel L, Peña FJ (2017) Computational flow cytometry reveals that cryopreservation induces spermptosis but a subpopulation of spermatozoa experiences capacitation like changes. Reproduction 153:293–304. https://doi.org/10.1530/REP-16-0359
Pothula SP, Xu Z, Goldstein D, Pirola RC, Wilson JS, Apte MV (2016) Key role of pancreatic stellate cells in pancreatic cancer. Cancer Lett 381:194–200. https://doi.org/10.1016/j.canlet.2015.10.035
Prado NJ, Egan Beňová T, Diez ER, Knezl V, Lipták B, Ponce Zumino AZ, Llamedo-Soria M, Szeiffová Bačová B, Miatello RM, Tribulová N (2019) Melatonin receptor activation protects against low potassium-induced ventricular fibrillation by preserving action potentials and connexin-43 topology in isolated rat hearts. J Pineal Res 67:e12605. https://doi.org/10.1111/jpi.12605
Roskoski R Jr (2016) Cyclin-dependent protein kinase inhibitors including palbociclib as anticancer drugs. Pharmacol Res 107:249–275. https://doi.org/10.1016/j.phrs.2016.03.012
Saitta B, Elphingstone J, Limfat S, Shkhyan R, Evseenko D (2019) CaMKII inhibition in human primary and pluripotent stem cell-derived chondrocytes modulates effects of TGFβ and BMP through SMAD signaling. Osteoarthr Cartil 27:158–171. https://doi.org/10.1016/j.joca.2018.08.017
Santofimia-Castaño P, Ruy DC, Salido GM, González A (2013) Melatonin modulates Ca2+ mobilization and amylase release in response to cholecystokinin octapeptide in mouse pancreatic acinar cells. J Physiol Biochem 69:897–908. https://doi.org/10.1007/s13105-013-0267-2
Santofimia-Castaño P, Salido GM, González A (2013) Ebselen alters mitochondrial physiology and reduces viability of rat hippocampal astrocytes. DNA Cell Biol 32:147–155. https://doi.org/10.1089/dna.2012.1939
Santofimia-Castaño P, Garcia-Sanchez L, Ruy DC, Sanchez-Correa B, Fernandez-Bermejo M, Tarazona R, Salido GM, Gonzalez A (2015) Melatonin induces calcium mobilization and influences cell proliferation independently of MT1/MT2 receptor activation in rat pancreatic stellate cells. Cell Biol Toxicol 31:95–110. https://doi.org/10.1007/s10565-015-9297-6
Shimada M, Andoh A, Hata K, Tasaki K, Araki Y, Fujiyama Y, Bamba T (2002) IL-6 secretion by human pancreatic periacinar myofibroblasts in response to inflammatory mediators. J Immunol 168:861–868. https://doi.org/10.4049/jimmunol.168.2.861
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT (2012) Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 351:152–166
Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidović D, Schürer SC, Xu J, Wagoner G, Drew PD, Griffin PR, Burris TP (2011) Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472:491–494. https://doi.org/10.1038/nature10075
Sonehara NM, Lacerda JZ, Jardim-Perassi BV, de Paula Jr R Jr, Moschetta-Pinheiro MG, Souza YST, de Andrade JCJ, De Campos Zuccari DAP (2019) Melatonin regulates tumor aggressiveness under acidosis condition in breast cancer cell lines. Oncol Lett 17:1635–1645. doi: https://doi.org/10.3892/ol.2018.9758
Song J, Ma SJ, Luo JH, Zhang H, Wang RX, Liu H, Li L, Zhang ZG, Zhou RX (2018) Melatonin induces the apoptosis and inhibits the proliferation of human gastric cancer cells via blockade of the AKT/MDM2 pathway. Oncol Rep 39:1975–1983. https://doi.org/10.3892/or.2018.6282
Sugden D, Yeh LK, Teh MT (1999) Design of subtype selective melatonin receptor agonists and antagonists. Reprod Nutr Dev 39:335–344. https://doi.org/10.1051/rnd:19990306
Tamtaji OR, Mirhosseini N, Reiter RJ, Behnamfar M, Asemi Z (2019) Melatonin and pancreatic cancer: current knowledge and future perspectives. J Cell Physiol 234:5372–5378. https://doi.org/10.1002/jcp.27372
Thakur N, Kumari S, Mehrotra R (2018) Association between Cyclin D1 G870A (rs9344) polymorphism and cancer risk in Indian population: meta-analysis and trial sequential analysis. Biosci Rep 38(6). https://doi.org/10.1042/BSR20180694
Tyagi N, Deshmukh SK, Srivastava SK, Azim S, Ahmad A, Al-Ghadhban A, Singh AP, Carter JE, Wang B, Singh S (2018) ETV4 facilitates cell-cycle progression in pancreatic cells through transcriptional regulation of cyclin D1. Mol Cancer Res 16:187–196. https://doi.org/10.1158/1541-7786.MCR-17-0219
Wang Y, Kumar N, Solt LA, Richardson TI, Helvering LM, Crumbley C, Garcia-Ordonez RD, Stayrook KR, Zhang X, Novick S, Chalmers MJ, Griffin PR, Burris TP (2010) Modulation of retinoic acid receptor-related orphan receptor alpha and gamma activity by 7-oxygenated sterol ligands. J Biol Chem 285:5013–5025. https://doi.org/10.1074/jbc.M109.080614
Wang Y, Keskanokwong T, Cheng J (2019) Kv4.3 expression abrogates and reverses norepinephrine-induced myocyte hypertrophy by CaMKII inhibition. J Mol Cell Cardiol 126:77–85. https://doi.org/10.1016/j.yjmcc.2018.11.011
Xi SC, Siu SW, Fong SW, Shiu SY (2001) Inhibition of androgen-sensitive LNCaP prostate cancer growth in vivo by melatonin: association of antiproliferative action of the pineal hormone with mt1 receptor protein expression. Prostate 46:52–61. https://doi.org/10.1002/1097-0045(200101)46:1<52::aid-pros1008>3.0.co;2-z
Yun M, Kim EO, Lee D, Kim JH, Kim J, Lee H, Lee J, Kim SH (2014) Melatonin sensitizes H1975 non-small-cell lung cancer cells harboring a T790M-targeted epidermal growth factor receptor mutation to the tyrosine kinase inhibitor gefitinib. Cell Physiol Biochem 34:865–8672. https://doi.org/10.1159/000366305
Zha M, Li F, Xu W, Chen B, Sun Z (2014) Isolation and characterization of islet stellate cells in rat. Islets 6:e28701. https://doi.org/10.4161/isl.28701
Acknowledgments
The authors would like to thank Mrs. Ana M. Moreno for her excellent technical support.
Funding
This study was partly funded by the Ministerio de Economía y Competitividad (BFU2016-79259-R), Ministerio de Ciencia, Innovación y Universidades (EQC2018-004646-P), and Junta de Extremadura-FEDER (IB16006; GR18070).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
- Melatonin, at pharmacological concentrations, modulates viability of PSC.
- Melatonin stimulates caspase-3 activation.
- Melatonin might regulate cell cycle in a cyclin-dependent manner.
- These actions might not involve specific melatonin receptors.
Rights and permissions
About this article
Cite this article
Estaras, M., Peña, F.J., Tapia, J.A. et al. Melatonin modulates proliferation of pancreatic stellate cells through caspase-3 activation and changes in cyclin A and D expression. J Physiol Biochem 76, 345–355 (2020). https://doi.org/10.1007/s13105-020-00740-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-020-00740-6