Abstract
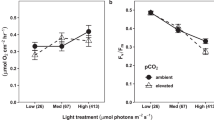
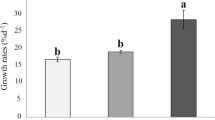
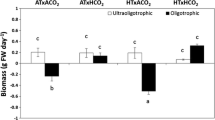
Although the adverse impacts of ocean acidification (OA) on marine calcifiers have been investigated extensively, the anti-stress capabilities regulated by increased light availability are unclear. Herein, the interactive effects of three light levels (30 µmol photons/(m2·s), 150 µmol photons/(m2·s), and 240 µmol photons/(m2·s) combined with two pCO2 concentrations (400 ppmv and 1 400 ppmv) on the physiological acclimation of the calcifying macroalga Halimeda opuntia were investigated using a pCO2-light coupling experiment. The OA negatively influenced algal growth, calcification, photosynthesis, and other physiological performances in H. opuntia. The relative growth rate under elevated pCO2 conditions significantly declined by 13.14%–41.29%, whereas net calcification rates decreased by nearly three-fold under OA conditions. Notably, increased light availability enhanced stress resistance through the accumulation of soluble organic molecules, especially soluble carbohydrate, soluble protein, and free amino acids, and in combination with metabolic enzyme-driven activities, OA stress was alleviated. The carotenoid content under low light conditions increased markedly, and the rapid light curve of the relative electron transport rate was enhanced significantly by increasing light intensities, indicating that this new organization of the photosynthetic machinery in H. opuntia accommodated light variations and elevated pCO2 conditions. Thus, the enhanced metabolic performance of the calcifying macroalga H. opuntia mitigated OA-related stress.
Similar content being viewed by others
References
Andersson A J, Kuffner I B, Mackenzie F T, et al. 2009. Net loss of CaCO3 from coral reef communities due to human induced seawater acidification. Biogeosciences Discussions, 6(1): 2163–2182
Bao Menglin, Wang Jianhao, Xu Tianpeng, et al. 2019. Rising CO2 levels alter the responses of the red macroalga Pyropia yezoensis under light stress. Aquaculture, 501: 325–330, doi: https://doi.org/10.1016/j.aquaculture.2018.11.011
Beach K, Walters L, Vroom P, et al. 2003. Variability in the ecophysiology of Halimeda spp. (Chlorophyta, Bryopsidales) on Conch Reef, Florida Keys, USA. Journal of Phycology, 39(4): 633–643, doi: https://doi.org/10.1046/j.1529-8817.2003.02147.x
Boardman N K. 1977. Comparative photosynthesis of sun and shade plants. Annual Review of Plant Physiology, 28: 355–377, doi: https://doi.org/10.1146/annurev.pp.28.060177.002035
Borowitzka M A, Larkum A W D. 1976. Calcification in the green alga Halimeda: III. The sources of inorganic carbon for photosynthesis and calcification and a model of the mechanism of calcification. Journal of Experimental Botany, 27(5): 879–893, doi: https://doi.org/10.1093/jxb/27.5.879
Caldeira K, Wickett M E. 2005. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. Journal of Geophysical Research, 110(C9): C09S04, doi: https://doi.org/10.1029/2004JC002671
Campbell J E, Fisch J, Langdon C, et al. 2016. Increased temperature mitigates the effects of ocean acidification in calcified green algae (Halimeda spp.). Coral Reefs, 35(1): 357–368, doi: https://doi.org/10.1007/s00338-015-1377-9
Celis-Plá P S M, Martínez B, Korbee N, et al. 2017. Photoprotective responses in a brown macroalgae Cystoseira tamariscifolia to increases in CO2 and temperature. Marine Environmental Research, 130: 157–165, doi: https://doi.org/10.1016/j.marenvres.2017.07.015
Chen Binbin, Zou Dinghui, Du Hong, et al. 2018. Carbon and nitrogen accumulation in the economic seaweed Gracilaria lemaneiformis affected by ocean acidification and increasing temperature. Aquaculture, 482: 176–182, doi: https://doi.org/10.1016/j.aquaculture.2017.09.042
Chen Binbin, Zou Dinghui, Ma Jiahai. 2016. Interactive effects of elevated CO2 and nitrogen- phosphorus supply on the physiological properties of Pyropia haitanensis (Bangiales, Rhodophyta). Journal of Applied Phycology, 28(2): 1235–1243, doi: https://doi.org/10.1007/s10811-015-0628-z
Chen Binbin, Zou Dinghui, Yang Yufeng. 2017. Increased iron availability resulting from increased CO2 enhances carbon and nitrogen metabolism in the economical marine red macroalga Pyropia haitanensis (Rhodophyta). Chemosphere, 173: 444–451, doi: https://doi.org/10.1016/j.chemosphere.2017.01.073
Chisholm J R M. 2000. Calcification by crustose coralline algae on the Northern Great Barrier Reef, Australia. Limnology and Oceanography, 45(7): 1476–1484, doi: https://doi.org/10.4319/lo.2000.45.7.1476
Chisholm J R M. 2003. Primary productivity of reef-building crustose coralline algae. Limnology and Oceanography, 48(4): 1376–1387, doi: https://doi.org/10.4319/lo.2003.48.4.1376
Comeau S, Edmunds P J, Lantz C A, et al. 2014a. Water flow modulates the response of coral reef communities to ocean acidification. Scientific Reports, 4: 6681
Comeau S, Edmunds P J, Spindel N B, et al. 2014b. Fast coral reef calcifiers are more sensitive to ocean acidification in short-term laboratory incubations. Limnology and Oceanography, 59(3): 1081–1091, doi: https://doi.org/10.4319/lo.2014.59.3.1081
Cornwall C E, Hepburn C D, McGraw C M, et al. 2013. Diurnal fluctuations in seawater pH influence the response of a calcifying macroalga to ocean acidification. Proceedings of the Royal Society B: Biological Sciences, 280(1772): 20132201, doi: https://doi.org/10.1098/rspb.2013.2201
Corzo A, Niell F X. 1991. Determination of nitrate reductase activity in Ulva rigida C. Agardh by the in situ method. Journal of Experimental Marine Biology and Ecology, 146(2): 181–191, doi: https://doi.org/10.1016/0022-0981(91)90024-Q
De Beer D, Larkum A W D. 2001. Photosynthesis and calcification in the calcifying algae Halimeda discoidea studied with microsensors. Plant, Cell & Environment, 24(11): 1209–1217
Dickson A G, Sabine C L, Christian J R. 2007. Guide to best practices for ocean CO2 measurements. Sidney: North Pacific Marine Science Organization, 67–81
Doney S C, Fabry V J, Feely R A, et al. 2009. Ocean acidification: the other CO2 problem. Annual Review of Marine Science, 1: 169–192, doi: https://doi.org/10.1146/annurev.marine.010908.163834
Doo S S, Edmunds P J, Carpenter R C. 2019. Ocean acidification effects on in situ coral reef metabolism. Scientific Reports, 9(1): 12067, doi: https://doi.org/10.1038/s41598-019-48407-7
Dupont S, Havenhand J, Thorndyke W, et al. 2008. Near-future level of CO2-driven ocean acidification radically affects larval survival and development in the brittlestar Ophiothrix fragilis. Marine Ecology Progress Series, 373: 285–294, doi: https://doi.org/10.3354/meps07800
Eilers P H C, Peeters J C H. 1988. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecological Modelling, 42(3–4): 199–215
El-Manawy I M, Shafik M A. 2008. Morphological characterization of Halimeda (Lamouroux) from different biotopes on the Red Sea coral reefs of Egypt. American-Eurasian Journal of Agricultural and Environmental Sciences, 3(4): 532–538
Elzenga J T M, Prins H B A. 1989. Light-induced polar pH changes in leaves of Elodea canadensis. I. Effects of carbon concentration and light intensity. Plant Physiology, 91(1): 62–67, doi: https://doi.org/10.1104/pp.91.1.62
Fabry V J, Seibel B A, Feely R A, et al. 2008. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Journal of Marine Science, 65(3): 414–432, doi: https://doi.org/10.1093/icesjms/fsn048
Feely R A, Sabine C L, Lee K, et al. 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science, 305(5682): 362–366, doi: https://doi.org/10.1126/science.1097329
Fougère F, Le Rudulier D, Streeter J G. 1991. Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiology, 96(4): 1228–1236, doi: https://doi.org/10.1104/pp.96.4.1228
Friedlingstein P, Jones M W, O’Sullivan M, et al. 2019. Global carbon budget 2019. Earth System Science Data, 11(4): 1783–1838, doi: https://doi.org/10.5194/essd-11-1783-2019
Haglund K, Björk M, Ramazanov Z, et al. 1992. Role of carbonic anhydrase in photosynthesis and inorganic-carbon assimilation in the red alga Gracilaria tenuistipitata. Planta, 187(2): 275–281
Hall-Spencer J M, Rodolfo-Metalpa R, Martin S, et al. 2008. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature, 454(7200): 96–99, doi: https://doi.org/10.1038/nature07051
Hemm M R, Rider S D, Ogas J, et al. 2004. Light induces phenylpropanoid metabolism in Arabidopsis roots. The Plant Journal, 38(5): 765–778, doi: https://doi.org/10.1111/j.1365-313X.2004.02089.x
Hillis L. 1997. Coralgal reefs from a calcareous green alga perspective, and a first carbonate budget. In: Proceedings of the 8th International Coral Reef Symposium. Panama: Balboa, 761–766
Hoegh-Guldberg O, Mumby P J, Hooten A J, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science, 318(5857): 1737–1742, doi: https://doi.org/10.1126/science.1152509
Hofmann L C, Heiden J, Bischof K, et al. 2014. Nutrient availability affects the response of the calcifying chlorophyte Halimeda opuntia (L.) J. V. Lamouroux to low pH. Planta, 239(1): 231–242, doi: https://doi.org/10.1007/s00425-013-1982-1
Hofmann G E, O’Donnell M J, Todgham A E. 2008. Using functional genomics to explore the effects of ocean acidification on calcifying marine organisms. Marine Ecology Progress Series, 373: 219–225, doi: https://doi.org/10.3354/meps07775
IPCC. 2013. Climate change 2013: the physical science basis. In: Stocker T F, Qin D H, Plattner G K, et al., eds. Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press, 1535
Kinsey D W, Hopley D. 1991. The significance of coral reefs as global carbon sinks-response to Greenhouse. Palaeogeography, Palaeoclimatology, Palaeoecology, 89(4): 363–377.
Koch M, Bowes G, Ross C, et al. 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology, 19(1): 103–132, doi: https://doi.org/10.1111/j.1365-2486.2012.02791.x
Kochert G. 1978a. Carbohydrate determination by phenol sulphuric acid method. In: Hellebust J A, Craigie J S, eds. Handbook of Phycological Methods: Physiological and Biochemical Methods. Cambridge: Cambridge University Press, 95–97
Kochert G. 1978b. Protein determination by dye binding. In: Hellebust J A, Craigie J S, eds. Handbook of Phycological Methods: Physiological and Biochemical Methods. Cambridge: Cambridge University Press, 91–93
Losada M, Guerrero M G. 1979. The photosynthetic reduction of nitrate and its regulation. In: Barber J, ed. Photosynthesis in Relation to Model Systems. Amsterdam: Elsevier, 365–408
Manzello D P. 2010. Coral growth with thermal stress and ocean acidification: lessons from the eastern tropical Pacific. Coral Reefs, 29(3): 749–758, doi: https://doi.org/10.1007/s00338-010-0623-4
Meyer F W, Schubert N, Diele K, et al. 2016. Effect of inorganic and organic carbon enrichments (DIC and DOC) on the photosynthesis and calcification rates of two calcifying green algae from a Caribbean reef lagoon. PLoS ONE, 11(8): e0160268, doi: https://doi.org/10.1371/journal.pone.0160268
Miedema H, Prins H B A. 1991. pH-dependent proton permeability of the plasma membrane is a regulating mechanism of polar transport through the submerged leaves of Potamogeton lucens. Canadian Journal of Botany, 69(5): 1116–1122, doi: https://doi.org/10.1139/b91-143
Milliman J D. 1993. Production and accumulation of calcium carbonate in the ocean: budget of a nonsteady state. Global Biogeochemical Cycles, 7(4): 927–957, doi: https://doi.org/10.1029/93GB02524
Moodley L, Boschker H T S, Middelburg J J, et al. 2000. Ecological significance of benthic foraminifera: 13C labelling experiments. Marine Ecology Progress Series, 202: 289–295, doi: https://doi.org/10.3354/meps202289
Morton B, Blackmore G. 2001. South China Sea. Marine Pollution Bulletin, 42(12): 1236–1263, doi: https://doi.org/10.1016/S0025-326X(01)00240-5
Moya A, Huisman L, Ball E E, et al. 2012. Whole transcriptome analysis of the coral Acropora millepora reveals complex responses to CO2-driven acidification during the initiation of calcification. Molecular Ecology, 21(10): 2440–2454, doi: https://doi.org/10.1111/j.1365-294X.2012.05554.x
O’Donnell M J, Todgham A E, Sewell M A, et al. 2010. Ocean acidification alters skeletogenesis and gene expression in larval sea urchins. Marine Ecology Progress Series, 398: 157–171, doi: https://doi.org/10.3354/meps08346
Orr J C, Fabry V J, Aumont O, et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature, 437(7059): 681–686, doi: https://doi.org/10.1038/nature04095
Payri C E. 1988. Halimeda contribution to organic and inorganic production in a Tahitian reef system. Coral Reefs, 6(3–4): 251–262
Peach K E, Koch M S, Blackwelder P L, et al. 2017. Calcification and photophysiology responses to elevated pCO2 in six Halimeda species from contrasting irradiance environments on Little Cayman Island reefs. Journal of Experimental Marine Biology and Ecology, 486: 114–126, doi: https://doi.org/10.1016/j.jembe.2016.09.008
Pierrot D, Lewis E, Wallace D W R. 2006. MS Excel program developed for CO2 system calculations: ORNL/CDIAC-105a. Oak Ridge, Tennessee: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U. S. Department of Energy
Porzio L, Buia M C, Hall-Spencer J M. 2011. Effects of ocean acidification on macroalgal communities. Journal of Experimental Marine Biology and Ecology, 400(1–2): 278–287
Price N N, Hamilton S L, Tootell J S, et al. 2011. Species-specific consequences of ocean acidification for the calcareous tropical green algae Halimeda. Marine Ecology Progress Series, 440: 67–78, doi: https://doi.org/10.3354/meps09309
Prins H B A, Snel J F H, Zanstra P E, et al. 1982. The mechanism of bicarbonate assimilation by the polar leaves of Potamogeton and Elodea. CO2 concentrations at the leaf surface. Plant, Cell & Environment, 5(3): 207–214
Rees S A, Opdyke B N, Wilson P A, et al. 2007. Significance of Halimeda bioherms to the global carbonate budget based on a geological sediment budget for the Northern Great Barrier Reef, Australia. Coral Reefs, 26: 177–188, doi: https://doi.org/10.1007/s00338-006-0166-x
Ries J B, Cohen A L, McCorkle D C. 2009. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology, 37(12): 1131–1134, doi: https://doi.org/10.1130/G30210A.1
Ries J B, Cohen A L, McCorkle D C. 2010. A nonlinear calcification response to CO2-induced ocean acidification by the coral Oculina arbuscula. Coral Reefs, 29(3): 661–674, doi: https://doi.org/10.1007/s00338-010-0632-3
Rizhsky L, Liang Hongjian, Shuman J, et al. 2004. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiology, 134(4): 1683–1696, doi: https://doi.org/10.1104/pp.103.033431
Sinutok S, Hill R, Doblin M A, et al. 2012. Microenvironmental changes support evidence of photosynthesis and calcification inhibition in Halimeda under ocean acidification and warming. Coral Reefs, 31(4): 1201–1213, doi: https://doi.org/10.1007/s00338-012-0952-6
Sun Yanguo, Wang Bo, Jin Shanghui, et al. 2013. Ectopic expression of Arabidopsis glycosyltransferase UGT85A5 enhances salt stress tolerance in tobacco. PLoS ONE, 8(3): e59924, doi: https://doi.org/10.1371/journal.pone.0059924
Sun Changqing, Yang Yanjun, Guo, Zhili, et al. 2015. Effects of fertilization and density on soluble sugar and protein and nitrate reductase of hybrid foxtail millet. Plant Nutrition and Fertilizer Science, 21(5): 1169–1177
Syrett P J. 1981. Nitrogen metabolism of microalgae. Canadian Journal of Fisheries and Aquatic Sciences, 210: 182–210
Teichberg M, Fricke A, Bischof K. 2013. Increased physiological performance of the calcifying green macroalga Halimeda opuntia in response to experimental nutrient enrichment on a Caribbean coral reef. Aquatic Botany, 104: 25–33, doi: https://doi.org/10.1016/j.aquabot.2012.09.010
Van Oijen T, Van Leeuwe M A, Gieskes W W C, et al. 2004. Effects of iron limitation on photosynthesis and carbohydrate metabolism in the Antarctic diatom Chaetoceros brevis (Bacillariophyceae). European Journal of Phycology, 39(2): 161–171, doi: https://doi.org/10.1080/0967026042000202127
Vásquez-Elizondo R M, Enríquez S. 2016. Coralline algal physiology is more adversely affected by elevated temperature than reduced pH. Scientific Reports, 6: 19030, doi: https://doi.org/10.1038/srep19030
Vásquez-Elizondo R M, Enríquez S. 2017. Light absorption in coralline algae (Rhodophyta): a morphological and functional approach to understanding species distribution in a coral reef lagoon. Frontiers in Marine Science, 4: 297, doi: https://doi.org/10.3389/fmars.2017.00297
Verma D P S. 1999. Osmotic stress tolerance in plants: role of praline and sulfur metabolisms. In: Shinozaki K, Yamaguchi-Shinozaki K, eds. Molecular Responses to Cold, Drought, Heat, and Salt Stress in Higher Plants. Austin: R. G. Landes Company, 153–168
Vidal-Dupiol J, Zoccola D, Tambutté E, et al. 2013. Genes related to ion-transport and energy production are upregulated in response to CO2-driven pH decrease in corals: new insights from transcriptome analysis. PLoS ONE, 8(3): e58652, doi: https://doi.org/10.1371/journal.pone.0058652
Wei Zhangliang, Long Chao, Yang Fangfang, et al. 2020a. Increased irradiance availability mitigates the physiological performance of species of the calcifying green macroalga Halimeda in response to ocean acidification. Algal Research, 48: 101906, doi: https://doi.org/10.1016/j.algal.2020.101906
Wei Zhangliang, Long Chao, Yang Fangfang, et al. 2020b. Effects of plant growth regulators on physiological performances of three calcifying green macroalgae Halimeda species (Bryopsidales, Chlorophyta). Aquatic Botany, 161: 103186, doi: https://doi.org/10.1016/j.aquabot.2019.103186
Wei Zhangliang, Mo Jiahao, Hu Qunju, et al. 2019. The physiological performance of the calcifying green macroalga Halimeda opuntia in response to ocean acidification with irradiance variability. Marine Science Bulletin, 38(5): 574–584
Wei Zhangliang, Zhang Yating, Yang Fangfang, et al. 2021. Increased light availability modulates carbon and nitrogen accumulation in the macroalga Gracilariopsis lemaneiformis (Rhodophyta) in response to ocean acidification. Environmental and Experimental Botany, 187: 104492, doi: https://doi.org/10.1016/j.envexpbot.2021.104492
Wellburn A R. 1994. The Spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology, 144(3): 307–313, doi: https://doi.org/10.1016/S0176-1617(11)81192-2
Wizemann A, Meyer F W, Hofmann L C, et al. 2015. Ocean acidification alters the calcareous microstructure of the green macroalga Halimeda opuntia. Coral Reefs, 34(3): 941–954, doi: https://doi.org/10.1007/s00338-015-1288-9
Xiong Liming, Schumaker K S, Zhu Jiankang. 2002. Cell signaling during cold, drought, and salt stress. The Plant Cell, 14(S1): S165–S183
Yang Qing, Liu Qiyong, Bai Yan, et al. 2009. Responses of cholorophyll and soluble protein in different leaf layers of winter wheat to N and P nutrients. Journal of Triticeae Crops, 29(1): 128–133
Zeebe R E, Zachos J C, Caldeira K, et al. 2008. Oceans. Carbon emissions and acidification. Science, 321(5885): 51–52, doi: https://doi.org/10.1126/science.1159124
Zou Dinghui, Gao Kunshan. 2010. Photosynthetic acclimation to different light levels in the brown marine macroalga, Hizikia fusiformis (Sargassaceae, Phaeophyta). Journal of Applied Phycology, 22(4): 395–404, doi: https://doi.org/10.1007/s10811-009-9471-4
Zou Dinghui, Gao Kunshan. 2013. Thermal acclimation of respiration and photosynthesis in the marine macroalga Gracilaria lemaneiformis (Gracilariales, Rhodophyta). Journal of Phycology, 49(1): 61–68, doi: https://doi.org/10.1111/jpy.12009
Acknowledgements
We thank the staff of Tropical Marine Biological Research Station in Hainan, Chinese Academy of Sciences for providing logistical support. Thanks are also due to editors and anonymous reviewers for their valuable comments and suggestions.
Funding
The National Natural Science Foundation of China under contract No. 42006129; the Guangzhou Science and Technology Project under contract No. 202102021228; the National Key Research and Development Project of China under contract No. 2021YFC3100500; the Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) under contract No. GML2019ZD0404; the Special Research Assistant Grant Program of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
13131_2022_2037_MOESM1_ESM.pdf
Increased light availability enhances tolerance against ocean acidification-related stress in the calcifying macroalga Halimeda opuntia
Rights and permissions
About this article
Cite this article
Wei, Z., Zhang, Y., Yang, F. et al. Increased light availability enhances tolerance against ocean acidification-related stress in the calcifying macroalga Halimeda opuntia. Acta Oceanol. Sin. 41, 123–132 (2022). https://doi.org/10.1007/s13131-022-2037-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13131-022-2037-x