Abstract
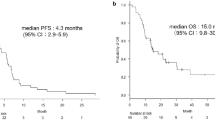
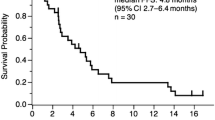
Bevacizumab (Bev), a monoclonal antibody against vascular endothelial growth factor, when combined with standard first-line chemotherapy, shows impressive clinical benefit in advanced non-squamous non-small cell lung cancer (ns-NSCLC). Our study aims to investigate whether the addition of Bev to pemetrexed improves progression-free survival (PFS) in advanced ns-NSCLC patients after the failure of at least one prior chemotherapy regimens. Patients with locally advanced, recurrent, or metastatic ns-NSCLC, after failure of platinum-based therapy, with a performance status 0 to 2, were eligible. Patients received 500 mg/m2 of pemetrexed intravenously (IV) day 1 with vitamin B12, folic acid, and dexamethasone and Bev 7.5 mg/kg IV day 1 of a 21-day cycle until unacceptable toxicity, disease progression or the patient requested therapy discontinuation. The primary end point was PFS. Between December 2011 and October 2013, 33 patients were enrolled, with median age of 55 years and 36.4 % men. Twenty-three patients (69.7 %) had received two or more prior regimens, and 28 patients (84.8 %) had received chemotherapy containing pemetrexed. The median number of the protocol regimens was 4. Median PFS was 4.37 months (95 % CI 2.64–6.09 months). Median overall survival (OS) was 15.83 months (95 % CI 10.52–21.15 months). Overall response rates were 6.45 %. Disease control rate was 54.84 %. No new safety signals were detected. No patient experienced drug-related deaths. The combination of Bev and pemetrexed every 21 days is effective in ns-NSCLC patients who failed of prior therapies with improved PFS. Toxicities are similar with historical data of these two agents and are tolerable. Our results may provide more a regimen containing Bev and pemetrexed for Chinese clinical practice in previously treated ns-NSCLC.


Similar content being viewed by others
References
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA: Cancer J Clin. 2005;55(1):10–30.
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2008;26(21):3543–51. doi:10.1200/JCO.2007.15.0375.
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–8. doi:10.1056/NEJMoa011954.
Kelly K, Crowley J, Bunn Jr PA, Presant CA, Grevstad PK, Moinpour CM, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol: Off J Am Soc Clin Oncol. 2001;19(13):3210–8.
Sandler AB, Nemunaitis J, Denham C, von Pawel J, Cormier Y, Gatzemeier U, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2000;18(1):122–30.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. doi:10.1056/NEJMoa0810699.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. doi:10.1056/NEJMoa0909530.
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–8. doi:10.1016/S1470-2045(09)70364-X.
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. doi:10.1016/s1470-2045(11)70393-x.
Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42. doi:10.1016/s1470-2045(11)70184-x.
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol: Off J Am Soc Clin Oncol. 2009;27(8):1227–34. doi:10.1200/JCO.2007.14.5466.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. doi:10.1056/NEJMoa061884.
Barlesi F, Scherpereel A, Rittmeyer A, Pazzola A, Ferrer Tur N, Kim JH, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol: Off J Am Soc Clin Oncol. 2013;31(24):3004–11. doi:10.1200/JCO.2012.42.3749.
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703. doi:10.1056/NEJMoa1006448.
Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. doi:10.1056/NEJMoa1214886.
Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372(9652):1809–18. doi:10.1016/S0140-6736(08)61758-4.
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol: Off J Am Soc Clin Oncol. 2000;18(10):2095–103.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–32. doi:10.1056/NEJMoa050753.
Sun JM, Lee KH, Kim SW, Lee DH, Min YJ, Yun HJ, et al. Gefitinib versus pemetrexed as second-line treatment in patients with nonsmall cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234–42. doi:10.1002/cncr.27630.
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol: Off J Am Soc Clin Oncol. 2004;22(9):1589–97. doi:10.1200/JCO.2004.08.163.
Garassino MC, Martelli O, Broggini M, Farina G, Veronese S, Rulli E, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14(10):981–8. doi:10.1016/s1470-2045(13)70310-3.
Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13(3):247–55. doi:10.1016/S1470-2045(12)70063-3.
Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374(9699):1432–40. doi:10.1016/S0140-6736(09)61497-5.
Scagliotti G, Brodowicz T, Shepherd FA, Zielinski C, Vansteenkiste J, Manegold C, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer. 2011;6(1):64–70. doi:10.1097/JTO.0b013e3181f7c6d4.
Margolin K, Gordon MS, Holmgren E, Gaudreault J, Novotny W, Fyfe G, et al. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol: Off J Am Soc Clin Oncol. 2001;19(3):851–6.
Gordon MS, Margolin K, Talpaz M, Sledge Jr GW, Holmgren E, Benjamin R, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2001;19(3):843–50.
Patel JD, Hensing TA, Rademaker A, Hart EM, Blum MG, Milton DT, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2009;27(20):3284–9. doi:10.1200/JCO.2008.20.8181.
Smit EF, Mattson K, von Pawel J, Manegold C, Clarke S, Postmus PE. ALIMTA (pemetrexed disodium) as second-line treatment of non-small-cell lung cancer: a phase II study. Ann Oncol: Off J Eur Soc Med Oncol / ESMO. 2003;14(3):455–60.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Nat Cancer Inst. 2000;92(3):205–16.
Fan Y, Huang ZY, Yu HF, Luo LH. Efficacy of salvage chemotherapy in the advanced non-small cell lung cancer patients who failed the treatment of chemotherapy and EGFR-TKI]. Zhonghua Zhong liu za zhi [Chinese Journal of Oncology]. 2010;32(11):859–63.
Dong L, Han ZF, Feng ZH, Jia ZY. Comparison of pemetrexed and docetaxel as salvage chemotherapy for the treatment for nonsmall-cell lung cancer after the failure of epidermal growth factor receptor-tyrosine kinase inhibitors. J Int Med Res. 2014;42(1):191–7. doi:10.1177/0300060513505808.
Loupakis F, Pollina L, Stasi I, Ruzzo A, Scartozzi M, Santini D, et al. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2009;27(16):2622–9. doi:10.1200/JCO.2008.20.2796.
Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. Bmj. 1995;311(7010):899–909.
Wachters FM, Groen HJ, Biesma B, Schramel FM, Postmus PE, Stigt JA, et al. A randomised phase II trial of docetaxel vs docetaxel and irinotecan in patients with stage IIIb-IV non-small-cell lung cancer who failed first-line treatment. Br J Cancer. 2005;92(1):15–20. doi:10.1038/sj.bjc.6602268.
Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol: Off J Am Soc Clin Oncol. 2000;18(12):2354–62.
Georgoulias V, Kouroussis C, Agelidou A, Boukovinas I, Palamidas P, Stavrinidis E, et al. Irinotecan plus gemcitabine vs irinotecan for the second-line treatment of patients with advanced non-small-cell lung cancer pretreated with docetaxel and cisplatin: a multicentre, randomised, phase II study. Br J Cancer. 2004;91(3):482–8. doi:10.1038/sj.bjc.6602010.
Takeda K, Negoro S, Tamura T, Nishiwaki Y, Kudoh S, Yokota S, et al. Phase III trial of docetaxel plus gemcitabine versus docetaxel in second-line treatment for non-small-cell lung cancer: results of a Japan Clinical Oncology Group trial (JCOG0104). Ann Oncol: Off J Eur Soc Med Oncol/ ESMO. 2009;20(5):835–41. doi:10.1093/annonc/mdn705.
Di Maio M, Chiodini P, Georgoulias V, Hatzidaki D, Takeda K, Wachters FM, et al. Meta-analysis of single-agent chemotherapy compared with combination chemotherapy as second-line treatment of advanced non-small-cell lung cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2009;27(11):1836–43. doi:10.1200/JCO.2008.17.5844.
Herbst RS, Sun Y, Eberhardt WE, Germonpre P, Saijo N, Zhou C, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11(7):619–26. doi:10.1016/S1470-2045(10)70132-7.
Herbst RS, Ansari R, Bustin F, Flynn P, Hart L, Otterson GA, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377(9780):1846–54. doi:10.1016/S0140-6736(11)60545-X.
Kim ES, Neubauer M, Cohn A, Schwartzberg L, Garbo L, Caton J, et al. Docetaxel or pemetrexed with or without cetuximab in recurrent or progressive non-small-cell lung cancer after platinum-based therapy: a phase 3, open-label, randomised trial. Lancet Oncol. 2013;14(13):1326–36. doi:10.1016/s1470-2045(13)70473-x.
Adjei AA, Mandrekar SJ, Dy GK, Molina JR, Adjei AA, Gandara DR, et al. Phase II trial of pemetrexed plus bevacizumab for second-line therapy of patients with advanced non-small-cell lung cancer: NCCTG and SWOG study N0426. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28(4):614–9. doi:10.1200/JCO.2009.23.6406.
Acknowledgments
We thank all the patients who entered this study. We acknowledge the contributions of staffs of the department of Medical Oncology, Sun Yat-Sen University Center for their assistance in either the conduct of this study or the preparation of this manuscript.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, L., Liu, K., Jiang, Z. et al. The efficacy and safety of pemetrexed plus bevacizumab in previously treated patients with advanced non-squamous non-small cell lung cancer (ns-NSCLC). Tumor Biol. 36, 2491–2499 (2015). https://doi.org/10.1007/s13277-014-2862-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2862-4