Abstract
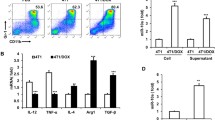
Tumour microenvironment is a key factor for cancer growth and metastasis. Tumour surrounding tissue is known to include high number of mesenchymal stem cells which have been thought to have a role in regulating cancer cell behaviour via paracrine signalling. Therefore, modulating human mesenchymal stem cell (hMSC) secretome is highly significant for controlling and treating disease. Since common therapeutic agents are known to enhance cancer resistance, there is a strong urge to define novel agents for developing cell-based therapies. In the present study, we aimed at investigating the effect of active compounds, myrtucommulone-A (MC-A) and thymoquinone (TQ), on hMSC cytokine expression. Our data revealed that MC-A treatment have significantly altered cytokine expression in hMSCs. Upon MC-A treatment, hMSCs decreased the expression levels of various cytokines including TNF-α, VEGF, IL-6, IL-8 and FGF-2. hMSC conditioned medium (CM) primed with MC-A decreased the proliferation, migration ability and clonogenicity of bladder cancer cells and breast cancer cells in comparison to non-primed hMSC medium and hMSC medium primed with TQ. To the best of our knowledge, this study is the first report showing the effects of active compounds, MC-A and TQ, on hMSCs and therefore valuable for highlighting the potential use of active compounds in combination with hMSCs for cell-based targeted cancer therapy.







Similar content being viewed by others
References
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–7.
Dorronsoro A, Fernández-Rueda J, Fechter K, Ferrin I, Salcedo JM, Jakobsson E, Trigueros C: Human mesenchymal stromal cell-mediated immunoregulation: mechanisms of action and clinical applications. Bone Marrow Res 2013;2013.
Ramdasi S, Sarang S, Viswanathan C. Potential of mesenchymal stem cell based application in cancer. Int J Hematol Oncol Stem Cell Res. 2015;9:95–103.
Yu JM, Jun ES, Bae YC, Jung JS. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17:463–73.
Tian LL, Yue W, Zhu F, Li S, Li W. Human mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vivo. J Cell Physiol. 2011;226:1860–7.
Luo J, Ok Lee S, Liang L, Huang CK, Li L, Wen S, et al. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene. 2014;33:2768–78.
Hou L, Wang X, Zhou Y, Ma H, Wang Z, He J, et al. Inhibitory effect and mechanism of mesenchymal stem cells on liver cancer cells. Tumour Biol. 2014;35:1239–50.
Atsuta I, Liu S, Miura Y, Akiyama K, Chen C, An Y, et al. Mesenchymal stem cells inhibit multiple myeloma cells via the Fas/Fas ligand pathway. Stem Cell Res Ther. 2013;4:111.
Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui KM, et al. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells. 2013;31:146–55.
Lis R, Touboul C, Raynaud CM, Malek JA, Suhre K, Mirshahi M, et al. Mesenchymal cell interaction with ovarian cancer cells triggers pro-metastatic properties. PLoS One. 2012;7, e38340.
Halpern JL, Kilbarger A, Lynch CC. Mesenchymal stem cells promote mammary cancer cell migration in vitro via the CXCR2 receptor. Cancer Lett. 2011;308:91–9.
Zhao M, Sachs PC, Wang X, Dumur CI, Idowu MO, Robila V, et al. Mesenchymal stem cells in mammary adipose tissue stimulate progression of breast cancer resembling the basal-type. Cancer Biol Ther. 2012;13:782–92.
Mandel K, Yang Y, Schambach A, Glage S, Otte A, Hass R. Mesenchymal stem cells directly interact with breast cancer cells and promote tumor cell growth in vitro and in vivo. Stem Cells Dev. 2013;22:3114–27.
Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F. Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–9.
Scherzed A, Hackenberg S, Froelich K, Kessler M, Koehler C, Hagen R, et al. Bmsc enhance the survival of paclitaxel treated squamous cell carcinoma cells in vitro. Cancer Biol Ther. 2011;11:349–57.
Chen DR, Lu DY, Lin HY, Yeh WL. Mesenchymal stem cell-induced doxorubicin resistance in triple negative breast cancer. Biomed Res Int. 2014;2014:532161.
Müller H, Paul M, Hartmann D, Huch V, Blaesius D, Koeberle A, et al. Total synthesis of myrtucommulone a. Angew Chem Int Ed Engl. 2010;49:2045–9.
Iskender B, Izgi K, Karaca H, Canatan H: Myrtucommulone-a treatment decreases pluripotency- and multipotency-associated marker expression in bladder cancer cell line HTB-9. J Nat Med 2015;[Epub ahead of print].
Izgi K, Iskender B, Jauch J, Sezen S, Cakir M, Charpentier M, Canatan H, Sakalar C: Myrtucommulone-a induces both extrinsic and intrinsic apoptotic pathways in cancer cells. J Biochem Mol Toxicol 2015;[Epub ahead of print].
Abukhader MM. Thymoquinone in the clinical treatment of cancer: fact or fiction? Pharmacogn Rev. 2013;14:117–20.
Sakalar C, Yuruk M, Kaya T, Aytekin M, Kuk S, Canatan H. Pronounced transcriptional regulation of apoptotic and TNF-NF-kappa-b signaling genes during the course of thymoquinone mediated apoptosis in HeLa cells. Mol Cell Biochem. 2013;383:243–51.
Darakhshan S, Bidmeshki PA, Hosseinzadeh CA, Sisakhtnezhad S. Thymoquinone and its therapeutic potentials. Pharmacol Res. 2015;95-96C:138–58.
Solchaga LA, Penick KJ, Welter JF: Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: Tips and tricks. Methods Mol Biol 2011;698.
Chen J, Crawford R, Chen C, Xiao Y. The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng Part B Rev. 2013;19:516–28.
Barcellos-de-Souza P, Gori V, Bambi F, Chiarugi P. Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta. 1836;2013:321–35.
Ye J, Wu D, Wu P, Chen Z, Huang J. The cancer stem cell niche: cross talk between cancer stem cells and their microenvironment. Tumour Biol. 2014;35:3945–51.
Xu Q, Wang L, Li H, Han Q, Li J, Qu X, et al. Mesenchymal stem cells play a potential role in regulating the establishment and maintenance of epithelial-mesenchymal transition in MCF7 human breast cancer cells by paracrine and induced autocrine TGF-β. Int J Oncol. 2012;41:959–68.
Ye H, Cheng J, Tang Y, Liu Z, Xu C, Liu Y, et al. Human bone marrow-derived mesenchymal stem cells produced TGFbeta contributes to progression and metastasis of prostate cancer. Cancer Invest. 2012;30:513–8.
Di GH, Liu Y, Lu Y, Liu J, Wu C, Duan HF. IL-6 secreted from senescent mesenchymal stem cells promotes proliferation and migration of breast cancer cells. PLoS One. 2014;9, e113572.
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63.
Chen DR, Lu DY, Lin HY, Yeh WL: Mesenchymal stem cell-induced doxorubicin resistance in triple negative breast cancer. Biomed Res Int 2014;2014.
Wang M, Cai J, Huang F, Zhu M, Zhang Q, Yang T, et al. Pre-treatment of human umbilical cord-derived mesenchymal stem cells with interleukin-6 abolishes their growth-promoting effect on gastric cancer cells. Int J Mol Med. 2015;35:367–75.
De Luca A, Lamura L, Gallo M, Maffia V, Normanno N. Mesenchymal stem cell-derived interleukin-6 and vascular endothelial growth factor promote breast cancer cell migration. J Cell Biochem. 2012;113:3363–70.
Ding G, Wang L, Xu H, Xu Z, Feng C, Ding Q, et al. Mesenchymal stem cells in prostate cancer have higher expressions of SDF-1, CXCR4 and VEGF. Gen Physiol Biophys. 2013;32:245–50.
Tu B, Du L, Fan QM, Tang Z, Tang TT. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 2012;325:80–8.
Lin JT, Wang JY, Chen MK, Chen HC, Chang TH, Su BW, et al. Colon cancer mesenchymal stem cells modulate the tumorigenicity of colon cancer through interleukin 6. Exp Cell Res. 2013;319:2216–29.
Huang F, Wang M, Yang T, Cai J, Zhang Q, Sun Z, et al. Gastric cancer-derived MSC-secreted PDGF-DD promotes gastric cancer progression. J Cancer Res Clin Oncol. 2014;140:1835–48.
Cheng J, Ye H, Liu Z, Xu C, Zhang Z, Liu Y, et al. Platelet-derived growth factor-bb accelerates prostate cancer growth by promoting the proliferation of mesenchymal stem cells. J Cell Biochem. 2013;114:1510–8.
Hogan NM, Joyce MR, Murphy JM, Barry FP, O'Brien T, Kerin MJ, et al. Impact of mesenchymal stem cell secreted PAI-1 on colon cancer cell migration and proliferation. Biochem Biophys Res Commun. 2013;435:574–9.
Moustakas A, Heldin CH. Induction of epithelial-mesenchymal transition by transforming growth factor β. Semin Cancer Biol. 2012;22:446–54.
Oyanagi J, Kojima N, Sato H, Higashi S, Kikuchi K, Sakai K, et al. Inhibition of transforming growth factor-β signaling potentiates tumor cell invasion into collagen matrix induced by fibroblast-derived hepatocyte growth factor. Exp Cell Res. 2014;326:267–79.
Daly RJ, Carrick N, Darbre PD. Progression to steroid autonomy is accompanied by altered sensitivity to growth factors in S115 mouse mammary tumour cells. J Steroid Biochem Mol Biol. 1995;54:21–9.
Rosendahl AH, Forsberg G. IGF-i and IGFBP-3 augment transforming growth factor-beta actions in human renal carcinoma cells. Kidney Int. 2006;70:1584–90.
Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappab activation. Nature. 2000;406:86–90.
Demarchi F, Bertoli C, Sandy P, Schneider C. Glycogen synthase kinase-3 beta regulates NF-kappa B1/p105 stability. J Biol Chem. 2003;278:39583–90.
Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86.
Graham JR, Tullai JW, Cooper GM. GSK-3 represses growth factor-inducible genes by inhibiting NF-kappaB in quiescent cells. J Biol Chem. 2010;285:4472–80.
Gong R, Rifai A, Ge Y, Chen S, Dworkin LD. Hepatocyte growth factor suppresses proinflammatory NFκB activation through GSK3β inactivation in renal tubular epithelial cells. J Biol Chem. 2008;283:7401–10.
Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281:9971–6.
Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32.
Cherla RP, Lee SY, Mulder RA, Lee MS, Tesh VL. Shiga toxin 1-induced proinflammatory cytokine production is regulated by the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling pathway. Infect Immun. 2009;77:3919–31.
Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84.
Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–77.
Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M, Zlabinger GJ, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–31.
Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280:25485–90.
Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45.
Yonezawa K, Tokunaga C, Oshiro N, Yoshino K. Raptor, a binding partner of target of rapamycin. Biochem Biophys Res Commun. 2004;313:437–41.
Wang L, Harris TE, Roth RA, Lawrence JCJ. PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–44.
Acknowledgments
We are grateful to Prof. Dr. Johann Jauch and Dr. Maël Charpentier (Institut für Organische Chemie der Universität des Saarlandes in Saarbrücken) for providing MC-A. This study was suppoted by the grants from the The Scientific and Technological Research Council of Turkey (No: 115S042, No: 114S542 and No: 113S927).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
The effects of conditioned media (hMSC CM, hMSC + MC-A CM, hMSC + TQ CM) on MDA-MB-231 clonogenicity and colony morphology. a Clonogenicity of highly aggressive human breast cancer cell line MDA-MB-231 was impaired upon hMSC + MC-A CM treatment. b The number of colonies formed was not affected from hMSC CM treatment in MDA-MB-231 cells as determined by measurement of the optical density of crystal violet staining. hMSC + TQ CM and hMSC + MC-A CM seemed to reduce clonogenicity of MDA-MB-231 cells. Error bars indicate mean ± SD (n = 2). *p < 0.05 vs MDA-MB-231 control; **p < 0.05 vs MDA-MB-231 control c MDA-MB-231 cells formed dispersed colonies and colony number but not colony morphology was affected from CM treatment. (PDF 8592 kb)
Rights and permissions
About this article
Cite this article
Iskender, B., Izgi, K., Sakalar, C. et al. Priming hMSCs with a putative anti-cancer compound, myrtucommulone-a: a way to harness hMSC cytokine expression via modulating PI3K/Akt pathway?. Tumor Biol. 37, 1967–1981 (2016). https://doi.org/10.1007/s13277-015-3995-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3995-9