Abstract
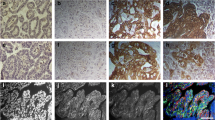
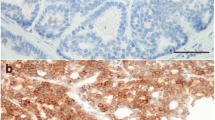
Considering that scarce data are available on disease progression of feline mammary carcinoma (FMC), this study aimed to analyze the clinical, pathological, and immunophenotypic features collected from 61 queens with FMC and to compare the concordance ratios of the expression levels of five molecular markers (ER, PR, fHER2, CK5/6, and Ki-67) between primary tumors (PT) and metastatic lesions. The results showed that cats with luminal A mammary carcinomas (MC) had higher overall survival (924.6 days, p = 0.001) and longer disease-free period (385.4 days, p = 0.005) compared to the ones with other MC subtypes. In fact, queens with triple negative/basal-like MC showed the lowest survival (mean 156.2 days) and the shortest disease-free survival (mean 28 days) among the molecular subtypes of MC. The lung was the organ most frequently affected by metastases, and animals with lung and/or pleural metastases were more likely to display metastases at three or more locations (p = 0.039). A large heterogeneity in protein expression levels was found between PT and paired metastases, with both estrogen and progesterone receptors more likely to be downregulated in metastases. Paired metastases frequently had higher Ki-67 index than PT, whereas fHER2 overexpression was seen in 46 samples (30 %) and CK5/6 expression was found in 50.7 % of metastases (36/71). Results also revealed that disease progression leads to a high percentage of triple negative/basal-like metastases (9/23; 39.1 %) associated with the absence of luminal A subtype in distant metastases (0/23). This study highlights the prognostic importance of immunophenotyping of MC in cats, although the modified protein expression identified in metastases contributes to justify why possible targeted therapies may fail in some animals with metastatic disease. Altogether, the results obtained also demonstrate that FMC can be used as a model to study human breast cancer.


Similar content being viewed by others
References
Sorenmo KU, Worley DR, Goldschmidt MH. Tumors of the mammary gland. In: Withrow, MacEwen’s, editors. Small animal clinical oncology. 5th ed. Missouri: Saunders Elsevier; 2013. p. 538–56.
Zappulli V, Rasotto R, Caliari D, Mainenti M, Peña L, Goldschmidt MH, et al. Prognostic evaluation of feline mammary carcinomas: a review of the literature. Vet Pathol. 2015;52:46–60.
Weigelt B, Glas AM, Wessels LFA, Witteveen AT, Peterse JL, van’t Veer LJ. Gene expression profiles of primary breast tumors maintained in distant metastases. PNAS. 2003;100:15901–5.
Montel V, Huang TY, Mose E, Pestonjamasp K, Tarin D. Expression profiling of primary tumors and matched lymphatic and lung metastases in a xenogeneic breast cancer model. Am J Pathol. 2005;166:1565–79.
Suzuki M, Tarin D. Gene expression profiling of human lymph node metastases and matched primary breast carcinomas: clinical implications. Mol Oncol. 2007;1:172–80.
Chan A, Morey A, Brown B, Hastrich D, Willsher P, Ingram D. A retrospective study investigating the rate of HER2 discordance between primary breast carcinoma and locoregional or metastatic disease. BMC Cancer. 2012;12:555.
Montel V, Mose ES, Tarin D. Tumor-stromal interactions reciprocally modulate gene expression patterns during carcinogenesis and metastasis. Int J Cancer. 2006;119:251–63.
Aitken SJ, Thomas JS, Langdon SP, Harrison DJ, Faratian D. Quantitative analysis of change in ER, PR and HER2 expression in primary breast cancer and paired nodal metastases. Ann Oncol. 2010;21:1254–61.
Aurilio G, Disalvatore D, Pruneri G, Bagnardi V, Viale G, Curigliano G, et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer. 2014;50:277–89.
Raica M, Cîmpean AM, Ceauşu RA, Fulga V, Nica C, Rudico L, et al. Hormone receptors and HER2 expression in primary breast carcinoma and corresponding lymph node metastasis: do we need both? Anticancer Res. 2014;34:1435–40.
Niikura N, Liu J, Hayashi N, Mittendorf EA, Gong Y, Palla SL, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30:593–9.
Falck AK, Fernö M, Bendahl PO, Rydén L. St Gallen molecular subtypes in primary breast cancer and matched lymph node metastases—aspects on distribution and prognosis for patients with luminal A tumours : results from a prospective randomised trial. BMC Cancer. 2013;13:558.
Yao ZX, Lu LJ, Wang RJ, Jin LB, Liu SC, Li HY, et al. Discordance and clinical significance of ER, PR and HER2 status between primary breast cancer and synchronous axillary lymph node metastasis. Med Oncol. 2014;31:798.
de las Mulas JM, van Niel M, Millán Y, Blankenstein MA, van Mil F, Misdorp W. Immunohistochemical analysis of estrogen receptors in feline mammary gland benign and malignant lesions: comparison with biochemical assay. Domest Anim Endocrinol. 2000;18:111–25.
Millanta F, Calandrella M, Citi S, Della Santa D, Poli A. Overexpression of HER-2 in feline invasive mammary carcinomas: na immunohistochemical survey and evaluation of its prognostic potential. Vet Pathol. 2005;42:30–4.
Millanta F, Calandrella M, Vannozzi I, Poli A. Steroid hormone receptors in normal, dysplastic and neoplastic feline mammary tissues and their prognostic significance. Vet Rec. 2006;158:821–4.
Rasotto R, Caliari D, Castagnaro M, Zanetti R, Zappulli V. An immunohistochemical study of HER-2 expression in feline mammary tumours. J Comp Pathol. 2011;144:170–9.
Seixas F, Palmeira C, Pires MA, Bento MJ, Lopes C. Grade is an independent prognostic factor for feline mammary carcinomas: a clinicopathological and survival analysis. Vet J. 2011;187:65–71.
Peñafiel-Verdu C, Buendia AJ, Navarro JA, Ramirez GA, Vilafranca M, Altimira J, et al. Reduced expression of E-cadherin and β-catenin and high expression of basal cytokeratins in feline mammary carcinomas with regional metastasis. Vet Pathol. 2012;49:979–87.
Soares M, Correia J, Rodrigues P, Simões M, de Matos A, Ferreira F. Feline HER2 protein expression levels and gene status in feline mammary carcinoma: optimization of immunohistochemistry (IHC) and in situ hybridization (ISH) techniques. Microscopy Microanalysis. 2013;19:1–7.
Maniscalco L, Iussich S, Martin de las Mulas J, Millán Y, Biolatti B, Sasaki N, et al. Activation of AKT in feline mammary carcinoma: a new prognostic factor for feline mammary tumours. Vet J. 2012;191(1):65–71.
Brunetti B, Asproni P, Beha G, Muscatello LV, Millanta F, Poli A, et al. Molecular phenotype in mammary tumours of queens: correlation between primary tumour and lymph node metastasis. J Comp Pathol. 2013;148:206–13.
Soares M, Correia J, Murta A, Ferreira F. Immunophenotyping of primary and metastatic lesions in feline mammary tumors—are they equal? Microscopy Microanalysis. 2013;19 Suppl 4:19–20.
Beha G, Muscatello LV, Brunetti B, Asproni P, Millanta F, Poli A, et al. Molecular phenotype of primary mammary tumors and distant metastases in female dogs and cats. J Comp Pathol. 2014;150:194–7.
Elston CW, Ellis IO. Assessment of histological grade. In: Rosen’s breast pathology. Philadelphia: Lippincott-Raven; 1998. p. 365–82.
Misdorp W. Tumors of the mammary gland. In: Tumors in Domestic Animals. Fourth Ed. Meuten DJ editor, Iowa: 2002. pp. 575–606.
Mills SW, Musil KM, Davies JL, Hendrick S, Duncan C, Jackson ML, Kidney B, Philibert H, Wobeser BK, Simko E. Prognostic value of histologic grading for feline mammary carcinoma: a retrospective survival analysis. Vet Pathol. 2014; 1–12.
Harvey JM, Clark GM, Osborne CK, Allred C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81.
Mohsin SK, Weiss H, Havighurst T, Clark GM, Berardo M, Roanh LD, et al. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Mod Pathol. 2004;17:1545–54.
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4014.
De Maria R, Olivero M, Iussich S, Nakaichi M, Murata T, Biolatti B, et al. Spontaneous feline mammary carcinoma is a model of HER2 overexpressing poor prognosis human breast cancer. Cancer Res. 2005;65:907–12.
Ordás J, Millán Y, Dios R, Reymundo C, de las Mula JM. Proto-oncogene HER-2 in normal, dysplastic and tumorous feline mammary glands: an immunohistochemical and chromogenic in situ hybridization study. BMC Cancer. 2007;7:179.
Santos S, Baptista C, Abreu RMV, Bastos E, Amorim I, Gut IG, et al. ERBB2 in cat mammary neoplasias disclosed a positive correlation between RNA and protein low expression levels: a model for erbB-2 negative human breast cancer. Plos One. 2013;8, e83673.
Soares M, Ribeiro R, Carvalho S, Peleteiro M, Correia J, Ferreira F. Ki-67 as a Prognostic Factor in Feline Mammary carcinoma: what is the optimal cutoff value? Veterinary Pathology. 2015.
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebnart M, Thürlimann B, et al. Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer. Ann Oncol. 2013;24:2206–23.
Cummings MC, Simpson PT, Reid LE, Jayanthan J, Skerman J, Song S, et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol. 2014;232:23–31.
Simmons C, Miller N, Geddie W, Gianfelice D, Oldfield M, Dranitsaris G, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol. 2009;20:1499–504.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312.
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134:48–72.
Kwast ABG, Voogd AC, Menke-Pluijmers MBE, Linn SC, Sonke GS, Kiemeney LA, et al. Prognostic factors for survival in metastatic breast cancer by hormone receptor status. Breast Cancer Res Treatment. 2014;145:503–11.
Yu K, Di G, Wu J, Lu J, Shen K, Liu G, et al. Breast cancer patients with estrogen receptor-negative/progesterone receptor-positive tumors: being younger and getting less benefit from adjuvant tamoxifen treatment. J Cancer Res Clin Oncol. 2008;134:1347–54.
Ng CH, Pathy NB, Taib NA, Mun KS, Rhodes A, Yip CG. The estrogen receptor negative-progesterone receptor positive breast carcinoma is a biological entity and not a technical artifact. Asian Pacific J Cancer Prev. 2012;13:1111–3.
Macfarlane R, Seal M, Speers C, Woods R, Masoudi H, Aparicio S, et al. Molecular alterations between the primary breast cancer and the subsequent locoregional/metastatic tumor. Oncologist. 2012;17:172–8.
Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–71.
Bosch A, Eroles P, Zaragoza R, Viña JR, Lluch A. Triple-negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev. 2010;36:206–15.
Joensuu K, Leidenius M, Kero M, Andersson LC, Horwitz KB, Heikkilä P. ER, PR, HER2, Ki-67 and Ck5 in early and late relapsing breast cancer—reduced CK 5 expression in metastases. Breast Cancer: Basic Clin Res. 2013;7:23–34.
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–83.
Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, et al. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by panel of four biomarkers using immunohistochemistry. Breast. 2012;21:50–7.
Pathmanathan N, Balleine RL. Ki67 and proliferation in breast cancer. J Clin Pathol. 2013;66:512–6.
Maniscalco L, Millán Y, Iussich S, Denina M, Sánchez-Céspedes R, Gattino F, et al. Activation of mammalian target of rapamycin (mTOR) in triple negative feline mammary carcinomas. BMC Vet Res. 2013;9:80.
Wiese DA, Thaiwong T, Yuzbasiyan-Gurkan V, Kiupel M. Feline mammary basal-like adenocarcinomas: a potential model for human triple-negative breast cancer (TNBC) with basal-like subtype. BMC Cancer. 2013;13:403.
Acknowledgments
This study was supported by “Fundação para a Ciência e Tecnologia” (FCT) through the project CIISA/UID/CVT/00276/2013 and the PhD fellowship (SFRH/BD/70720/2010). The authors would like to thank João Matos and José Cabeçadas (MD) from Instituto Português de Oncologia de Lisboa (IPO); Manuel Mestre (DVM), Ana Mota (DVM, MSc) and Tiago Rafael (DVM, MSc) from the Clínica Veterinária Zoomédica; Mafalda Lage (DVM, MSc) from the Clínica Veterinária Villa Animal; Rafaela Lalanda (DVM, MSc) and Miguel Caninhas (DVM) from the Clínica Veterinária Mvet; Verónica Azevedo (DVM, MSc) from the Hospital Sul do Tejo; and António Ferreira (DVM, PhD), Ana Murta (DVM, MSc) and Rodrigo Bom (DVM) from the Small Animal Hospital from the Faculty of Veterinary Medicine at the University of Lisbon, for the clinical follow-up. We would also like to thank Margarida Simões (DVM, MSc) and Shabir Najmudin (DSc, PhD) from the Faculty of Veterinary Medicine at the University of Lisbon for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Tumor samples were collected in accordance with the EU Directive 2010/63/EU, and research was approved by the ethics committee of the Faculty of Veterinary Medicine (FVM), University of Lisbon (ULisboa).
Conflicts of interest
None
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 23 kb)
Supplementary Table 2
(DOCX 43 kb)
Supplementary Table 3
(DOCX 43 kb)
Supplementary Table 4
(DOCX 23 kb)
Supplementary Table 5
(DOCX 22 kb)
Supplementary Table 6
(DOCX 35 kb)
Supplementary Table 7
(DOCX 34 kb)
Supplementary Table 8
(DOCX 20 kb)
Supplementary Table 9
(DOCX 20 kb)
Supplementary Table 10
(DOCX 30 kb)
Supplementary Table 11
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Soares, M., Correia, J., Peleteiro, M.C. et al. St Gallen molecular subtypes in feline mammary carcinoma and paired metastases—disease progression and clinical implications from a 3-year follow-up study. Tumor Biol. 37, 4053–4064 (2016). https://doi.org/10.1007/s13277-015-4251-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4251-z