Abstract
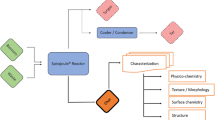
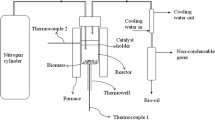
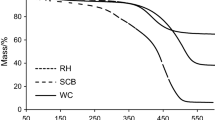
The thermochemical biomass conversion has widely used because it is considered environmentally friendly and carried out at low temperatures and pressure to reduce costs. Hydrothermal and pyrolysis carbonization are two kinds of biomass conversion that widely practiced in the current time. In this study, hydrothermal and pyrolysis carbonization was carried out using sago wastes in bark and pith form. The purpose of this research was to compare the charcoal properties between chars from hydrothermal and pyrolysis carbonization. In this study, the carbonization process was conducted in two methods, i.e., hydrothermal and pyrolysis carbonization. The hydrothermal carbonization was performed with a temperature of 250 °C for 4 h, using rotary digester with water as a medium. While the pyrolysis carbonization was conducted with a temperature of 400 °C for 5 h, using a vacuum electrically heated tube as a reactor. The characterization of chars performed was proximate and elemental analysis; iodine number; calorific value; Fourier-transform infrared (FTIR); X-ray diffraction (XRD); scanning electron microscope (SEM); Brunauer, Emmett, and Teller (BET); and pyrolysis-gas chromatography-mass spectroscopy (Pyr-GC-MS). The results showed that the iodine adsorption capacity, surface area, and pore volume of chars from hydrothermal were higher than pyrolysis. In contrast, the calorific value of chars from pyrolysis was higher than the chars from hydrothermal. Based on results, chars from hydrothermal can be used as a precursor of activated charcoal specifically for adsorbent, while chars from pyrolysis are potential energy sources.




Similar content being viewed by others
References
Ehara H, Toyoda Y, Johnson D (2018) Sago palm: multiple contributions to food security and sustainable livelihoods. Springer, Singapore
McClatchey W, Manner HI, Elevitch CR (2006) Metroxylon amicarum, M. paulcoxii, M. sagu, M. salomonense, M. vitiense, and M. warburgii (sago palm). Tradit Trees Pacific Islands Their Cult Environ Use Perm Agric Resour Holualoa, Hawai ‘i 2:491–512
Siruru H, Syafii W, Wistara INJ, Pari G (2019) Characteristics of Metroxylon rumphii (Pith and bark waste) from Seram Island, Maluku, Indonesia. Biodiversitas 20:3517–3526. https://doi.org/10.13057/biodiv/d201208
Johnravindar D, Murugesan K, Wong JWC, Elangovan N (2017) Waste-to-biofuel: production of biobutanol from sago waste residues. Environ Technol (United Kingdom) 38:1725–1734. https://doi.org/10.1080/09593330.2017.1283362
Neethu A, Murugan A (2018) Bioconversion of sago effluent and oil cakes for bio-butanol production using environmental isolates. Biofuels 0:1–8. https://doi.org/10.1080/17597269.2018.1446576
Basu P (2013) Biomass gasification, pyrolysis, and Torrefaction practical design and theory, 2nd ed. Elsevier Inc.
Hernandez-Mena LE, Pecora AAB, Beraldo AL (2014) Slow pyrolysis of bamboo biomass: analysis of biochar properties. Chem Eng Trans 37:115–120. https://doi.org/10.3303/CET1437020
Darmawan S (2014) Nanoporous carbon derived from forest biomass through the staged carbonization: pyrolysis, hydrothermal, and activation. Dissertation
Dai L, Tan F, Wu B, He M, Wang W, Tang X, Hu Q, Zhang M (2015) Immobilization of phosphorus in cow manure during hydrothermal carbonization. J Environ Manag 157:49–53. https://doi.org/10.1016/j.jenvman.2015.04.009
Heilmann SM, Molde JS, Timler JG, Wood BM, Mikula AL, Vozhdayev GV, Colosky EC, Spokas KA, Valentas KJ (2014) Phosphorus reclamation through hydrothermal carbonization of animal manures. Environ Sci Technol 48:10323–10329. https://doi.org/10.1021/es501872k
Ekpo U, Ross AB, Camargo-Valero MA, Williams PT (2016) A comparison of product yields and inorganic content in process streams following thermal hydrolysis and hydrothermal processing of microalgae, manure, and digestate. Bioresour Technol 200:951–960. https://doi.org/10.1016/j.biortech.2015.11.018
Zhang S, Zhu X, Zhou S, et al. (2018) Hydrothermal carbonization for hydrochar production and its application. Elsevier Inc.
Titirici MM, White RJ, Falco C, Sevilla M (2012) Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage. Energy Environ Sci 5:6796–6822. https://doi.org/10.1039/c2ee21166a
Sharma R, Jasrotia K, Singh N, Ghosh P, srivastava S, Sharma NR, Singh J, Kanwar R, Kumar A (2020) A comprehensive review on hydrothermal carbonization of biomass and its applications. Chem Africa 3:1–19. https://doi.org/10.1007/s42250-019-00098-3
Parshetti GK, Kent Hoekman S, Balasubramanian R (2013) Chemical, structural and combustion characteristics of carbonaceous products obtained by hydrothermal carbonization of palm empty fruit bunches. Bioresour Technol 135:683–689. https://doi.org/10.1016/j.biortech.2012.09.042
Ekpo U, Ross AB, Camargo-Valero MA, Fletcher LA (2016) Influence of pH on hydrothermal treatment of swine manure: impact on extraction of nitrogen and phosphorus in process water. Bioresour Technol 214:637–644. https://doi.org/10.1016/j.biortech.2016.05.012
Schneider (2011) Characterization of biochar from hydrothermal carbonization of bamboo. Int J Energy Environ 2:647–652
Hu B, Yu S-H, Wang K, Liu L, Xu XW (2008) PERSPECTIVE www.rsc.org/dalton | Dalton Transactions View Article Online Functional carbonaceous materials from hydrothermal carbonization of biomass: an effective chemical process. J Chem Soc Dalton Trans 9226:5414–5423. https://doi.org/10.1039/b804644c
Yoshimura M, Byrappa K (2008) Hydrothermal processing of materials: past, present, and future. J Mater Sci 43:2085–2103. https://doi.org/10.1007/s10853-007-1853-x
Indonesian National Standardization Agency Indonesian National Standard 06-3730-1995 for Technical Activated Charcoal
ASTM (2014) ASTM D4607-14 - Standard Method for Determination of Iodine Number of Activated Carbon
Kang S, Li X, Fan J, Chang J (2012) Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, d-xylose, and wood meal. Ind Eng Chem Res 51:9023–9031. https://doi.org/10.1021/ie300565d
Cao X, Zhong L, Peng X, Sun S, Li S, Liu S, Sun R (2014) Comparative study of the pyrolysis of lignocellulose and its major components: characterization and overall distribution of their biochars and volatiles. Bioresour Technol 155:21–27. https://doi.org/10.1016/j.biortech.2013.12.006
Lavoie JM, Baré W, Bilodeau M (2011) Depolymerization of steam-treated lignin for the production of green chemicals. Bioresour Technol 102:4917–4920. https://doi.org/10.1016/j.biortech.2011.01.010
Libra JA, Ro KS, Kammann C, Funke A, Berge ND, Neubauer Y, Titirici MM, Fühner C, Bens O, Kern J, Emmerich KH (2011) Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes, and applications of wet and dry pyrolysis. Biofuels 2:71–106. https://doi.org/10.4155/bfs.10.81
Wu X, Ba Y, Wang X, Niu M, Fang K (2018) Evolved gas analysis and slow pyrolysis mechanism of bamboo by thermogravimetric analysis, Fourier transform infrared spectroscopy and gas chromatography-mass spectrometry. Bioresour Technol 266:407–412. https://doi.org/10.1016/j.biortech.2018.07.005
Wu YM, Zhao ZL, Bin LH, He F (2009) Low temperature pyrolysis characteristics of major components of biomass. Ranliao Huaxue Xuebao/J Fuel Chem Technol 37:427–432. https://doi.org/10.1016/s1872-5813(10)60002-3
Sevilla M, Fuertes AB (2009) The production of carbon materials by hydrothermal carbonization of cellulose. Carbon N Y 47:2281–2289. https://doi.org/10.1016/j.carbon.2009.04.026
Tripathi M, Sahu JN, Ganesan P (2016) Effect of process parameters on production of biochar from biomass waste through pyrolysis: a review. Renew Sust Energ Rev 55:467–481. https://doi.org/10.1016/j.rser.2015.10.122
Mumme J, Eckervogt L, Pielert J, Diakité M, Rupp F, Kern J (2011) Hydrothermal carbonization of anaerobically digested maize silage. Bioresour Technol 102:9255–9260. https://doi.org/10.1016/j.biortech.2011.06.099
Nizamuddin S, Jayakumar NS, Sahu JN, Ganesan P, Bhutto AW, Mubarak NM (2015) Hydrothermal carbonization of oil palm shell. Korean J Chem Eng 32:1789–1797. https://doi.org/10.1007/s11814-014-0376-9
Funke A, Ziegler F (2010) Hydrothermal carbonization of biomass: a summary and discussion of chemical mecha- nisms for process engineering. Biofuels Bioprod Biorefin 4:160–177. https://doi.org/10.1002/bbb.198
Xu Y, Chen B (2013) Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour Technol 146:485–493. https://doi.org/10.1016/j.biortech.2013.07.086
Sevilla M, Fuertes AB (2009) Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem - A Eur J 15:4195–4203. https://doi.org/10.1002/chem.200802097
Lv GJ, Wu S, Bin, Lou R (2010) Characteristics of corn stalk hemicellulose pyrolysis in a tubular reactor. BioResources 5:2051–2062. https://doi.org/10.15376/biores.5.4.2051-2062
Gao Y, Chen HP, Wang J et al (2011) Characterization of products from hydrothermal liquefaction and carbonation of biomass model compounds and real biomass. Ranliao Huaxue Xuebao/J Fuel Chem Technol 39:893–900. https://doi.org/10.1016/S1872-5813(12)60001-2
Budiman I (2019) Development of self-healing mortar made from oil palm shell and oil palm empty fruit bunches. Dissertation
Song W, Guo M (2012) Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J Anal Appl Pyrolysis 94:138–145. https://doi.org/10.1016/j.jaap.2011.11.018
Budiman I, Hermawan D, Febrianto F, Pari G, Subyakto (2019) Char properties and pollutant adsorption capability of oil palm shell using hydrothermal process. Biomass Convers Biorefinery 9(4):681–688. https://doi.org/10.1007/s13399-019-00394-5
Mann JD (2012) Comparison of yield, calorific value and ash content in woody and herbaceous biomass used for bioenergy production in southern Ontario, Canada
Lee Y, Eum PRB, Ryu C, Park YK, Jung JH, Hyun S (2013) Characteristics of biochar produced from slow pyrolysis of Geodae-Uksae 1. Bioresour Technol 130:345–350. https://doi.org/10.1016/j.biortech.2012.12.012
Jindo K, Mizumoto H, Sawada Y, Sanchez-Monedero MA, Sonoki T (2014) Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 11:6613–6621. https://doi.org/10.5194/bg-11-6613-2014
Saeid A, Prochownik E, Dobrowolska-Iwanek J (2018) Phosphorus solubilization by Bacillus species. Molecules 23:1–18. https://doi.org/10.3390/molecules23112897
Enders A, Hanley K, Whitman T, Joseph S, Lehmann J (2012) Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour Technol 114:644–653. https://doi.org/10.1016/j.biortech.2012.03.022
Xiao LP, Shi ZJ, Xu F, Sun RC (2012) Hydrothermal carbonization of lignocellulosic biomass. Bioresour Technol 118:619–623. https://doi.org/10.1016/j.biortech.2012.05.060
Bardestani R, Kaliaguine S (2018) Steam activation and mild air oxidation of vacuum pyrolysis biochar. Biomass Bioenergy 108:101–112. https://doi.org/10.1016/j.biombioe.2017.10.011
Mettler MS, Vlachos DG, Dauenhauer PJ (2012) Top ten fundamental challenges of biomass pyrolysis for biofuels. Energy Environ Sci 5:7797–7809. https://doi.org/10.1039/c2ee21679e
Demirbas A (2005) Pyrolysis of ground beech wood in irregular heating rate conditions. J Anal Appl Pyrolysis 73:39–43. https://doi.org/10.1016/j.jaap.2004.04.002
Sonibare OO, Haeger T, Foley SF (2010) Structural characterization of Nigerian coals by X-ray diffraction, Raman, and FTIR spectroscopy. Energy 35:5347–5353. https://doi.org/10.1016/j.energy.2010.07.025
Marsh H, Rodríguez-Reinoso F (2006) Activated carbon. Act Carbon https://doi.org/10.1016/B978-0-08-044463-5.X5013-4
Funding
This research is financially supported by The Postgraduate Scholarship Program of the Ministry of Research, Technology and Higher Education of the Republic of Indonesia, and Research Institute of Pattimura University, Ambon, Maluku Province, Republic of Indonesia.
Author information
Authors and Affiliations
Contributions
All authors contributed to this work and discussed the results and implications and commented on the manuscript. Herman Siruru, Wasrin Syafii, I Nyoman J Wistara, and Gustan Pari act as the main contributors responsible for designing and formulating research methods, sample testing, research results, writing, and revising manuscript drafts, while Ismail Budiman acts as a member contributor responsible for the analysis and interpretation of research data, as well as writing and revising the manuscript drafts.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siruru, H., Syafii, W., Wistara, I.N.J. et al. Properties of sago waste charcoal using hydrothermal and pyrolysis carbonization. Biomass Conv. Bioref. 12, 5543–5554 (2022). https://doi.org/10.1007/s13399-020-00983-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00983-9