Abstract
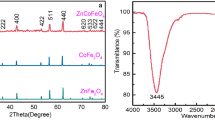
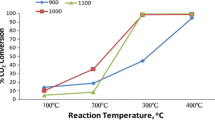
A series of cobalt-iron mixed oxides, CoxFe3−xO4 (x = 0; 0.05; 0.1; 0.15), were synthesized by coprecipitation and tested for oxidative decarboxylation of malic acid to pyruvic or malonic acid. The characterization of catalysts was performed by different techniques such as X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFT) and Ultraviolet–visible spectroscopy (UV–Vis). Among studied catalysts, Co0.15Fe2.85O4 sample (denoted Co3Fe) showed the highest malic acid conversion in oxidative decarboxylation reaction as well as the highest pyruvic acid yield. This behavior can be due to the fact that this sample has the highest content of tetrahedral Co2+ that replaces Fe3+ from octahedral position that determine an increased number of defects that play a crucial role for the malic acid conversion.














Similar content being viewed by others
References
Kövilein A, Kubisch C, Cai L, Ochsenreither K (2020) Malic acid production from renewables: a Review. J Chem Technol Biotechnol 95:513–526. https://doi.org/10.1002/jctb.6269
Zhang Z, Wang B, Zhou P, Guo D, Kang R, Zhang B (2016) A novel approach of chemical mechanical polishing using environment-friendly slurry for mercury cadmium telluride semiconductors. Nat Sci Rep 6:22466. https://doi.org/10.1038/srep22466
Bizzarri R, Chiellini F, Solaro R, Chiellini E, Cammas-Marion S, Guerin P (2002) Synthesis and characterization of new malolactonate polymers and copolymers for biomedical applications. Macromolecules 35:1215–1223. https://doi.org/10.1021/ma0111257
Aldrich D, Vink W, Deptula RW, Muskus DJ, Fronczkowski PR, Chrusch M (1979) Sugarless candies. US Patent 4:154:867, https://patents.google.com/patent/DK555978A
Bharathiraja B, Selvakumari IAE, Jayamuthunagai J, Kumar RP, Varjani S, Pandey A, Gnansounou E (2020) Biochemical conversion of biodiesel by-product into malic acid: a way towards sustainability. Sci Total Environ 709:136206. https://doi.org/10.1016/j.scitotenv.2019.136206
Song Y, Li JH, Shin HD, Liu L, Du GC, Chen J (2016) Biotechnological production of alpha-keto acids: current status and perspectives. Bioresour Technol 219:716–724. https://doi.org/10.1016/j.biortech.2016.08.015
Luo Z, Yu S, Zeng W, Zhou J (2021) Comparative analysis of the chemical and biochemical synthesis of keto acids. Biotechnol Adv 47:107706. https://doi.org/10.1016/j.biotechadv.2021.107706
Lomate S, Bonnotte T, Paul S, Dumeignil F, Katryniok B (2013) Synthesis of pyruvic acid by vapour phase catalytic oxidative dehydrogenation of lactic acid. J Mol Catal A: Chem 377:123–128. https://doi.org/10.1016/j.molcata.2013.05.006
Pal D, Keshav A, Mazumdar B, Kumar A, Uslu H (2017) Production and recovery of pyruvic acid: recent advances. J Inst Eng India Ser E 98:165–175. https://doi.org/10.1007/s40034-017-0101-4
Hou Y, Hossain GS, Li JH, Shin HD, Du GC, Liu L (2016) Combination of phenylpyruvic acid (PPA) pathway engineering and molecular engineering of L-amino acid deaminase improves PPA production with an Escherichia coli whole-cell biocatalyst. Appl Microbiol Biol 100:2183–2191. https://doi.org/10.1007/s00253-015-7048-5
Yin C, Li X, Dai Y, Chen Z, Dingfeng Y, Liu R, Zou W, Tang C, Dong L (2021) Facet-regulated oxidative dehydrogenation of lactic acid to pyruvic acid on α-Fe2O3. Green Chem 23:328–332. https://doi.org/10.1039/D0GC03468A
Lee H, Lee E, Kim DK, Jang NK, Jeong YY, Jon S (2006) Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. J Am Chem Soc 128(22):7383–7389. https://doi.org/10.1021/ja061529k
Zhu K, Jin C, Zhao C, Hu R, Klencsáre Z, Sundaram GA, Srankó DF, Ge R, Wang J (2019) Modulation synthesis of multi-shelled cobalt-iron oxides as efficient catalysts for peroxymonosulfate-mediated organics degradation. Chem Eng J 359:1537–1549. https://doi.org/10.1016/j.cej.2018.11.018
Xiong Y, Zhao J, Zheng Z, Li W (2020) Effect of copper dopant on the mixed cobalt-iron oxides for hydrogen generation via chemical looping redox cycles. Int J Hydrogen Energy 45:28372–28382. https://doi.org/10.1016/j.ijhydene.2020.07.245
Ren X, Lyu F, Yang J, Wang F, Xue L, Wang L, Zhang X, Wang Q (2019) Homogeneous cobalt and iron oxide hollow nanocages derived from ZIF-67 etched by Fe species for enhanced water oxidation. Electrochim Acta 296:418–426. https://doi.org/10.1016/j.electacta.2018.11.024
Hdidou L, Kouisni L, Manoun B, Hannache H, Solhy A, Barakat A (2018) Oxidative conversion of lignin over cobalt-iron mixed oxides prepared via the alginate gelation. Catal Commun 117:99–104. https://doi.org/10.1016/j.catcom.2018.08.027
Block T, Knoblauch N, Schmücker M (2014) The cobalt-oxide/iron-oxide binary system for use as high temperature thermochemical energy storage material. Thermochim Acta 577:25–32. https://doi.org/10.1016/j.tca.2013.11.025
Manjula N, Vinothkumar V, Chen SM (2021) Synthesis and characterization of iron-cobalt oxide/polypyrrole nanocomposite: an electrochemical sensing platform of anti-prostate cancer drug flutamide in human urine and serum samples. Colloids Surf A Physicochem Eng Asp 628:127367. https://doi.org/10.1016/j.colsurfa.2021.127367
Rossman GR (1996) Why hematite is red: correlation of optical absorption intensities and magnetic moments of Fe3+ minerals. Mineral Spectroscopy 5:023–028 Eds: Dyar MD, McCammon Schaefer CMW. https://www.geochemsoc.org/files/6214/1270/1006/SP-5_023-028_Rossman.pdf
Djemai A, Calas G, Muller JP (2001) Role of structural Fe(III) and iron oxide nanophases in mullite coloration. J Am Ceram Soc 84(7):1627–1631. https://doi.org/10.1111/j.1151-2916.2001.tb00887.x
Montenero A, Friggeri M, Giori DC, Belkhiria N, Pye LD (1986) Iron-soda-silica glasses: preparation, properties, structure. J Non-Cryst Solids 84:45–60. https://doi.org/10.1016/0022-3093(86)90761-1
Rada S, Dehelean A, Culea E (2011) FTIR, Raman, and UV-Vis spectroscopic and DFT investigations of the structure of iron–lead–tellurate glasses. J Mol Model 17:2103–2111. https://doi.org/10.1007/s00894-010-0911-5
Thota S, Kumar A, Kumar J (2009) Optical, electrical and magnetic properties of Co3O4 nanocrystallites obtained by thermal decomposition of sol-gel derived oxalates. Mat Sci Engin B 164:30–37. https://doi.org/10.1016/j.mseb.2009.06.002
Garcia MA, Jiménez-Villacorta F, Quesada A, de la Venta J, Carmona N, Lorite I, Llopis J, Fernández JF (2010) Surface magnetism in ZnO/Co3O4 mixtures. J Appl Phys 107:043906. https://doi.org/10.1063/1.3294649
Jian Y, Yu T, Jiang Z, Yu Y, Douthwaite M, Liu J, Albilali R, He C (2019) In-depth understanding of the morphology effect of α-Fe2O3 on catalytic ethane destruction. ACS Appl Mater Interfaces 11:11369–11383. https://doi.org/10.1021/acsami.8b21521
Natile MM, Glisenti A (2003) New NiO/Co3O4 and Fe2O3/Co3O4 nanocomposite catalysts: synthesis and characterization. Chem Mater 15:2502–2510. https://doi.org/10.1021/cm031019e
Galvita V, Rihko-Struckmann LK, Sundmacher K (2008) The CO adsorption on a Fe2O3–Ce0.5Zr0.5O2 catalyst studied by TPD, isotope exchange and FTIR spectroscopy. J Mol Catal A: Chem 283:43–51. https://doi.org/10.1016/j.molcata.2007.11.016
Cocivera M, Kokesh FC, Malatesta V, Zinck JJ (1977) Catalysis of keto-enol tautomerism of oxaloacetic acid and its ions studied by proton nuclear magnetic resonance. J Org Chem 42:4076. https://doi.org/10.1021/jo00445a019
Schiering DW, Katon JE (1986) The vibrational spectra and structure of oxaloacetic acid. J Mol Structure 144:71–82. https://doi.org/10.1016/0022-2860(86)80168-5
Liu Z, Li X, Ying P, Feng Z, Li C (2007) Fourier transform infrared spectroscopic study on the adsorption of ethyl pyruvate on Pt/Al2O3: side reactions suppressed by adsorbed hydrogen and cinchonidine. J Phys Chem C 111:823–829. https://doi.org/10.1021/jp064561+
Maroń MK, Takahashi K, Shoemaker RK, Vaida V (2011) Hydration of pyruvic acid to its geminal-diol, 2,2-dihydroxypropanoic acid, in a water-restricted environment. Chem Phys Letters 513:184–190. https://doi.org/10.1016/j.cplett.2011.07.090
Max JJ, Chapados C (2002) Infrared spectroscopy of aqueous carboxylic acids: malic acid. J Phys Chem A 106:6452–6461. https://doi.org/10.1021/jp014377i
Mansour H, Letifi H, Bargougui R, De Almeida-Didry S, Negulescu B, Autret-Lambert C, Gadri A, Ammar S (2017) Structural, optical, magnetic and electrical properties of hematite (α-Fe2O3) nanoparticles synthesized by two methods: polyols and precipitation. Appl Phys A 123:787. https://doi.org/10.1007/s00339-017-1408-1
de Faria DLA, Venâncio SS, de Oliveira MT (1997) Raman microspectroscopy of some iron oxides and oxyhydroxides. J Raman Spectrosc 28:873–878. https://doi.org/10.1002/(SICI)1097-4555(199711)28:11%3c873::AID-JRS177%3e3.0.CO;2-B
Jorand SC, Alexer BD, Solarska R, Rutkowska IA, Augustynski J, Cerny R (2005) Photoelectrochemical oxidation of water at transparent ferric oxide film electrodes. J Phys Chem B 109:13685–13692. https://doi.org/10.1021/jp051546g
Zhang X, Niu Y, Meng X, Li Y, Zhao J (2013) Structural evolution and characteristics of the phase transformations between α-Fe2O3, Fe3O4 and γ-Fe2O3 nanoparticles under reducing and oxidizing atmospheres. CrystEngComm 15:8166–8172. https://doi.org/10.1039/C3CE41269E
Pérez LC, Kado L, Zhang M, Müller AHE (2004) In situ laser-induced formation of α-Fe2O3 from Fe3+ ions in a cylindrical core-shell polymer brush. J Raman Spectrosc 35:165–169. https://doi.org/10.1002/jrs.1125
Herrmann-Geppert I, Bogdanoff P, Radnik J, Fengler S, Dittrich T, Fiechter S (2013) Surface aspects of sol–gel derived hematite films for the photoelectrochemical oxidation of water. Phys Chem Chem Phys 15:1389. https://doi.org/10.1039/C2CP42651J
Buga MR, Spinu-Zaulet AA, Ungureanu CG, Mitran RA, Vasile E, Florea M, Neatu F (2021) Carbon-coated SiO2 composites as promising anode material for Li-ion batteries. Molecules 26:4531. https://doi.org/10.3390/molecules26154531
Biesinger MC, Payne BP, Grosvenor AP, Lau LW, Gerson A, Smart R (2010) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl Surf Sci 257(7):2717–2730. https://doi.org/10.1016/j.apsusc.2010.10.051
Wang JC, Ren J, Yao HC, Zhang L, Wang JS, Zang SQ, Han LF, Li ZJ (2016) 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction. J Hazardous Materials 311:11–19. https://doi.org/10.1016/j.apcatb.2020.119051
Stadnichenko AI, Kibis LS, Svintsitskiy DA, Koshcheev SV, Boronin AI (2018) Application of RF discharge in oxygen to create highly oxidized metal layers. Surf Engin 34:1–5. https://doi.org/10.1179/1743294415Y.0000000010
Drif A, Pineda A, Morvan D, Belliere-Baca V, De Oliveira Vigier K, Jérôme F (2019) Catalytic oxidative dehydrogenation of malic acid to oxaloacetic acid. Green Chem 21(17):4604–4608. https://doi.org/10.1039/C9GC01768B
Liu J, Du Z, Yang Y, Lu T, Lu F, Xu J (2012) Catalytic oxidative decarboxylation of malic acid into dimethyl malonate in methanol with dioxygen. Chemsuschem 5:2151–2154. https://doi.org/10.1002/cssc.201200489
Neidig ML, Brown CD, Kavana M, Choroba OW, Spencer JB, Moran GR, Solomon EI (2006) Spectroscopic and electronic structure studies of the role of active site interactions in the decarboxylation reaction of α-keto acid-dependent dioxygenases. J Inorg Biochem 100:2108–2116. https://doi.org/10.1016/j.jinorgbio.2006.08.021
Ferretto L, Glisenti A (2002) Study of the surface acidity of a hematite powder. J Mol Catal A Chem 187:119–128. https://doi.org/10.1016/S1381-1169(02)00126-7
Gittus OR, von Rudorff GF, Rosso KM, Blumberger J (2018) Acidity constants of the hematite–liquid water interface from Ab initio molecular dynamics. J Phys Chem Lett 9(18):5574–5582. https://doi.org/10.1021/acs.jpclett.8b01870
Funding
This work was supported by a grant from the Romanian Ministry of Education and Research, CNCS–UEFISCDI, project number PNIII-P4-ID-PCE-2020–0580, within PNCDI III. Furthermore, M. Florea and F. Neatu were supported by Core Program PN19-03 (contract no. 21 N/08.02.2019).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Materials preparation was performed by Gheorghita Mitran, formal analysis and investigation by Gheorghita Mitran and Adriana Urda, materials characterization was performed by Mihaela Florea, Octavian Dumitru Pavel, Stefan Neatu, and Florentina Neatu. The first draft of the manuscript was written by Gheorghita Mitran and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mitran, G., Urdă, A., Pavel, OD. et al. A green way for pyruvic acid synthesis from biomass-derived L-malic acid on tetrahedral versus octahedral cobalt sites/hematite. Biomass Conv. Bioref. 14, 813–824 (2024). https://doi.org/10.1007/s13399-022-02513-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02513-1