Abstract
Polyhydroxyalkanoate (PHA) production at a large scale by purple phototrophic bacteria (PPB) is hindered due to high production costs and limited recovery due to its consumption during starvation periods. The present study identified cost-effective inorganic acids as inactivation methods for PPB to obtain higher PHA recovery. The study was performed on reactors of different scales (10 L and 0.5 L) to grow PPB and recover PHA subsequently. The permanent feast strategy was adopted to obtain higher PHA in an anaerobic environment. As a result, the study achieved 33% (dry weight) PHA recovery using inorganic acid inactivation, while formaldehyde inactivation (traditional method) achieved significantly lower PHA recovery (20% only). The results from inorganic acid inactivation were further examined for their stability. The samples were stable even after day 14, and the PHA recovery was the same as on day 0. This pioneering study shows that inorganic acids can be used to inactivate the PPB metabolism to obtain higher PHA recovery; inorganic acid inactivation could be economical for large-scale PHA production.
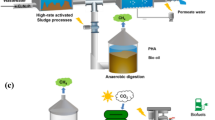
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbon-based fossil fuels are the primary resource for obtaining chemicals and energy. However, its dramatic depletion is shifting focus toward a bio-based circular economy [1]. A search for new resources, technologies, and strategies is being made to support a bio-based circular economy, with a prime focus on reducing dependency on carbon-based fossil fuels [2]. One of the major problems in the current scenario is petro-based plastics. Petroleum-based plastics are durable and low-cost and have good mechanical and thermal properties. However, their environmental impact is enormous as they are not degradable and can be in the environment for more than a century. Therefore, the search for a sustainable alternative for bio and circular economy is on the urge. Microorganisms as biocatalysts for bioremediation, production of biodegradable plastics, biofuels, and bioenergy can be excellent alternatives to promote sustainable development goals without impacting the environment [3]. Bioplastics have emerged as a sustainable alternative in this context, as they can be produced from renewable resources; some of them are biodegradable and have similar thermal and mechanical properties to petroleum-based plastics [3]. Naturally occurring polyhydroxyalkanoates (PHA) are aliphatic polymers, completely biodegradable, home and industrial compostable, and environmentally safe bioplastics [4, 5]. PHA have similar properties to petroleum-based plastics, such as thermoplastic and elastomeric properties, making them compatible for commercial applications. In addition, some microorganisms produce PHA as energy reserves during stress conditions, such as limited nutrients and light availability [3]. The synthesis of PHA depends on the type of carbon source and cultivation methods, metabolic pathway, and types of monomers incorporated by microorganisms.
Purple phototrophic bacteria (PPB) are versatile microorganisms that can grow in diverse environments in anaerobic and aerobic conditions. They have low energy requirements, high growth yield and low maintenance costs, and a wide range of cellular and metabolic products of value. PPB can convert waste sources such as wastewater into high-value products, for instance, bio-hydrogen, bioplastics, biofuels, and proteins [6]. The organics and nutrients in wastewaters are valuable resources for product recovery within the circular economy. PPB can perform anoxygenic photosynthesis using diverse wastewater sources to store PHA. In anoxic environments, PPB get favorable conditions to maintain their internal redox balance since they do not access other electron acceptors such as oxygen or nitrate, and they take carbon, which activates their metabolism to store PHA [3]. However, it is essential to maintain specific conditions to increase the accumulation of PHA; it is stated that the feast-famine strategy allows higher PHA accumulation in PPB. In the feast-famine strategy, PPB are first appropriately fed; in the second phase, the feeding is stopped. Due to stress conditions in the second phase, they accumulate PHA [7]. However, in an anaerobic environment, permanent feast (PF) strategy allows for higher PHA accumulation, where PPB are continuously fed with carbon in low concentration of other nutrients such as nitrogen (N) and phosphorus (P) [8]. Allegue et al. (2022) have recently reported advanced conditions for PPB growth to accumulate higher PHA; they associated steam explosion pretreatment and thermophilic acidogenic fermentation to the organic fraction of municipal solid waste (OFMSW) with organic loading rate (OLR) of 1 g COD L−1 d−1 and sludge and hydraulic retention times of 6 and 2 days, respectively, for stable growth of PPB to accumulate PHA.
Several companies have already adopted PHA production on a large scale using aerobic bacteria. However, high production costs are still significant, so commercialization is still not widespread [9, 10]. Major expenses in the production of PHA are determined by the fermentation substrate’s cost and the polymer’s extraction from inside the cell [11]. In the situation when PPB experience a shortage of substrates, they consume PHAs as a source of redox power, energy, and carbon. Thus, fixing the PPB biomass immediately after biomass collection is necessary to stop their metabolism and diminish the loss of PHA in bioplastic biorefineries. Formaldehyde is primarily used as a reagent to fix bacterial biomass and stop the assimilation and consumption of PHA before recovery [7, 12]. However, organic solvents like formaldehyde are expensive and non-environment-friendly. Hence, several chemical compounds, such as acid, alkali, oxidants, and surfactants, can be used to recover PHA from biomass by cell disintegration [13]. The concentration and amount of oxidants and alkali compounds are crucial to be optimized; otherwise, if these compounds increase, they degrade intracellular polymers, leading to PHA loss [14, 15]. However, using inorganic acids such as sulfuric acid (H2SO4), hydrochloric acid (HCl), and nitric acid (HNO3) can reduce the cost of PHA recovery and does not degrade PHA like alkali compounds [13]. Thus, this study aims to explore an economical and eco-friendlier solvent as an inactivating agent to stop PPB metabolism. The study’s primary goal was to maximize the PPB growth at a bench scale and achieve maximum PHA. The PPB were grown in two reactors: small (0.5 L) and big (10 L) glass bottles. The 0.5-L reactor was used as a control to compare with the PPB growth in 10 L. The inactivation experiments were performed with three inorganic acids, H2SO4, HCl, and HNO3, and were compared with the traditional cell deactivation method using formaldehyde solution. Further, a stability test for PHA recovery using different inorganic acids was also performed to evaluate the potential PHA degradation upon 14 days.
2 Material and methods
2.1 Inoculum and wastewater
The inoculum was taken from a naturally illuminated, 350-L, pilot-scale, 20-cm deep raceway pond closed with a UV–visible filter, supplemented with acetic acid, ammonium, and phosphorus as a substrate, operated under a hydraulic retention time of 3 days, and a solid retention time of 9 days. The pilot raceway pond is located at University Rey Juan Carlos, Mostoles, Spain (Fig. 1). The inoculum’s volatile suspended solids (VSS) were 100 mg L−1. The reactors were fed with municipal wastewater from the University Rey Juan Carlos, Mostoles, Spain, supplemented with different concentrations of acetic acid, 99.5% GLR, purchased from Labkem (Madrid, Spain). As a result, the wastewater contained soluble chemical oxygen demand (SCOD) of 168 ± 30 mg L−1, total COD (TCOD) of 187 ± 15 mg L−1, NH4+-N concentration of 52 ± 10 mg L−1, and phosphate (PO43−) concentration of 5 ± 4 mg L−1.
2.2 Operation of reactors
The experiment was performed in two open reactors made up of glass bottles: the first reactor as an upscaling reactor had a working volume of 10 L (R1) and the second as a reference with a volume of 0.5 L (R2). The R1 was illuminated by four Infrared (IR) lights (IR130, C&M Vision Technologies Inc., Houston, TX, USA) from all sides of the reactor. Similarly, R2 was illuminated by two IR lights from both sides. R1 (10 L) and R2 (0.5L) had a similar configuration. Figure 2a shows a schematic view of the reactor setup in the laboratory. The reactors were continuously stirred on a magnetic stirrer (IKA* C-MAG MS10, Staufen, Germany) at 80 rpm. For the proper growth of PPB in the laboratory environment, reactors were monitored for approximately two months to enrich PPB before the main experiment started. The temperature of both reactors was uncontrolled and varied at 24 ± 4 °C, and dissolved oxygen concentration was < 0.2 mg L−1 throughout the experiment. In addition, the pH of both reactors was monitored through a pH controller (Kontrol OI, ProsystemAqua, Cordoba, Spain) and maintained constant at pH 7 during the experiment. The reactors were operated in batch mode, where each batch lasted for 5 days, and three successive batch experiments were performed.
2.3 PPB enrichment and feeding strategy
Carbon and nutrients in adequate ratios are essential for PPB growth; in general, PPB are fed with high organics and low N and P ratios for higher PHA accumulation [8]. Therefore, a COD:N:P ratio of 100:3:0.5 was used (nutrient-limited conditions) for adequate PHA accumulation. Firstly, both reactors were fed with wastewater and acetic acid to maintain a COD:N:P ratio of 100:3:0.5. Then, an additional spike of acetic acid (1 g COD L−1) was added once all the nutrients were consumed during the batch. Figure 2b represents the overall feeding strategy of the batch. The initial soluble chemical oxygen demand (SCOD) was 1 g COD L−1. In anaerobic conditions, PPB can be grown in permanent feast conditions to have continuous availability of volatile fatty acids [8]; therefore, similar conditions were provided in the present study. Once the PPB mixed culture consumed the SCOD and nutrients, both reactors were spiked with 1 g COD L−1 of acetic acid, generally on the 3rd day of each batch. On the other hand, since the consumption of nutrients was slower in R1, the COD spike was unnecessary during the first two batches. The batch’s refreshment was performed using 10% volume from the previous batch. The reactors were cleaned daily by scrubbing the reactor walls to control light diffusion limitations.
2.4 Wastewater analysis
The wastewater parameters were monitored from day 1 to day 5 for each batch. The wastewater was monitored for COD, NH4+, PO43−, total suspended solids (TSS), and volatile suspended solids (VSS) at the start of each batch and after every 24 h during 5 days. The liquid samples were filtered through a cellulose filter of 0.45 μm pore size before analysis. COD, TSS, and VSS were analyzed according to APHA Standard Methods [16]. NH4+ and PO43− were analyzed through SmartChem140 (AMS ALLIANCE, Frepillon, France). A pH meter monitored pH and Oxidation Reduction Potential (ORP) daily (GLP 22, Crison, Spain). Temperature and dissolved oxygen (DO) were monitored by Hach portable dissolved oxygen meter (Hach, Colorado, USA). The PPB growth was monitored by scanning the absorbance of the biomass between 400 and 1100 nm wavelength using a UV Spectrophotometer (Agilent Technology, Santa Clara, CA, USA) and taking absorbance at wavelengths 660 nm (which is used for measuring optical density) and 805 and 865 nm (corresponding to the absorbance peaks of bacteriochlorophyll a that is typically found in purple phototrophic bacteria). The apparent biomass yield (Yx/s, in mgVSS mg COD−1) was calculated by the ratio between total biomass growth (VSS) and the total SCOD consumed.
2.5 Standard PHA extraction and analysis
The PHA extraction was done from 50 mL of fermentation broth (biomass suspension) taken from the reactors and immediately inactivated with 0.2% formaldehyde, followed by centrifugation and lyophilization (Standard M, Harvest Right, Spain) to prevent PHA degradation. The samples were further mixed with chloroform, and acid solution for PHA extraction following a protocol is described elsewhere [1]. PHA production was analyzed by gas chromatography using an HP-5 Agilent column and coupled with an FID detector (Agilent Technologies, Santa Clara, CA, USA). The column was initially maintained at 50 °C for 3 min, followed by 180 °C at 12 °C min−1, and maintained at 250 °C for 5 min at 7 °C min−1. Helium was used as carrier gas (1 mL min−1), and the injector temperature was maintained at 320 °C. The complete protocol for the analysis is described elsewhere [1]. Commercial standards of PHA were used to make calibration curves: one containing 88% of hydroxybutyrate (HB) and 12% of hydroxyvalerate (HV) and another with 100% methyl 3-hydroxyhexanoate (Sigma-Aldrich, USA). The PHA production was calculated as wt.% of dried biomass (d.b.) and expressed as PHA content/fraction (mPHA) by using Eq. 1.
2.6 Alternative PHA inactivation and stabilization
An inactivation method was used to make PHA production eco-friendlier and more economical than the standard one using formaldehyde as an inactivating agent. The PHA inactivation was performed using different inorganic acids to identify the best inactivating agent. The inactivation protocol with formaldehyde from Sect. 2.5 was considered the control method. The alternative inactivation was performed with three different inorganic acids (1 M): HCl, HNO3, and H2SO4. Three different pH (1, 2, and 3) and three time frames (1, 4, and 8 h) were considered influencing factors. Based on the operational parameters, a D-optimal randomized factorial with design model 2-factor interaction was performed using Design-Expert 12.0 software (Stat-Ease, Minnesota, MIN, USA). The total number of runs was 28 based on three different acids, pH, temperature, and 5-level replicate points.
For inactivation experiments, 6 L of volume from the R1 reactor was centrifuged at 5430 rcf for 15 min to reach at least 3 g TSS L−1 of biomass. Afterward, a jar test was performed with a working volume of 200 mL in each beaker. The pH of all runs during the jar test was controlled by a pH controller (ProsystemAqua, Cordoba, Spain). Samples were taken at the mentioned time interval and kept at 4 °C. Then, the samples were centrifuged, followed by the same PHA extraction and analysis process mentioned in Sect. 2.5. The measured concentration of PHA was then compared to the concentration of the control sample to determine the PHA recovery efficiency of the alternative inactivation method.
The stability of the PHA over time was measured by analyzing the PHA concentration on the same day of the experiment (expressed as day 0). The same sample was kept in a desiccator at room temperature and analyzed after 1 week (day 7), followed by the second week (day 14). The results from day 0 were compared with those from days 7 and 14. The results were considered stable if the PHA concentration after 2 weeks were not changed (at 95% confidence).
2.7 Data handling
Confidence intervals at 95% were calculated from triplicate measurements based on a two-tailed t-test from parameter standard error and used for statistically representative comparisons.
3 Results and discussions
3.1 Performance of PPB culture for accumulating PHA
The COD and nutrient consumption were monitored to observe PPB growth and PHA accumulation. Figure 3 shows the COD, N, and P concentration and removal during each batch experiment. The TCOD was relatively constant throughout the batch, and SCOD reduced with time in both reactors (Fig. 3a, b). The TCOD generally increases or stays constant when the biomass increases, whereas SCOD is consumed by biomass for its growth; thus, it decreases with time. Since PPB under phototrophic conditions are assimilative organisms, they do not reduce TCOD but convert it from soluble to particulate (assimilative growth) [17]. The SCOD consumption rate in R1 was 0.78 mgCOD mgCOD-biomass−1 d−1 at the start of the initial batch, which increased with time to 2.3 mgCOD mgCOD-biomass−1 d−1 at the end of the batch. On the other hand, in R2, the SCOD consumption rate at the initial batch was 1.3 mgCOD mgCOD-biomass−1 d−1, which decreased at the end of each batch to 0.8 mgCOD mgCOD-biomass−1 d−1. Therefore, R2 evidenced a higher SCOD consumption in the initial phases, resulting in a higher biomass yield than R1. However, the consumption rate was vice versa at the later stage resulting in higher biomass in R1. It also indicates that carbon was used for PPB biomass growth at the initial stages and was then majorly utilized for PHA accumulation rather than growth [8]. During initial batches, the nutrients’ consumption was faster in the R2 than in the R1 due to the higher volumetric irradiance (16 vs. 1.44 W L−1). Thus, it caused a concomitant faster PPB growth. Nonetheless, at the later stage, R1 and R2 performed similarly (Fig. 3), indicating that the irradiance had a low influence on the cultures’ growth. Figures 3a and 2b show that the R1 and R2 showed a sharp increment in TCOD after the COD spike during each batch. As stated in the literature, acetic acid promotes PPB growth, and an excess of organic carbon allows PHA accumulation irrespective of the nitrogen concentration, even during growth [18]. It is also generally stated that PHA-accumulating bacteria can grow in a two-stage process where they enrich in the first stage and accumulate PHA at high organic load and limited nutrient conditions [2, 19].
The PPB growth was monitored by measuring TSS and VSS daily. Similarly, the absorbance at 600 nm and 805 nm was also monitored to observe PPB growth. Figure 3e, f represents the suspended solids and absorbance ratio, respectively. The absorbance ratio (805/660 nm) increased after the COD spike, achieving values close to 1, indicating that the mixed microbial culture was highly enriched in PPB [20, 21].
It is mentioned in several studies that the PF regime (continuous presence of organic carbon) is a better way to produce high PHA in anaerobic conditions; therefore, in this study, the second COD spike had a significant role in biomass growth and PHA production [1, 8]. In the PF regime, PPB store PHA as an electron sink to stabilize the cell’s internal redox state [22]. Indeed, PPB can maintain PHA accumulation after entering in a resting (not-growing) state, as shown in the increment of the PHA accumulation shown, e.g., at day 16 after the second acetate spike in both reactors. Three samples per batch were taken to monitor PHA production based on the PPB growth (checking the absorbance at 660 nm). The PHA accumulation was higher in the R2 than in R1. The PHA mass fraction in biomass (wt.%, d.b.) in R1 at the end of batch 1, batch 2, and batch 3 was 17 ± 1%, 19 ± 1%, and 20 ± 1%, respectively. Similarly, the PHA accumulation from R2 in batch 1, batch 2, and batch 3 was 36 ± 3%, 31 ± 4%, and 31 ± 1%, respectively (Fig. 4). The PHA generally lowered on the 2nd and 3rd days; then, it increased with biomass growth until day 5 of each batch. Based on previous studies, one of the scale-up challenges for PHA production by PPB is the control of the PHA evolution. Indeed, on a large scale, PHA production is usually limited [2, 8]. PHA produced by PPB does not stay stable with time; it usually gets utilized by PPB themselves when the volumetric irradiance is not in excess, as in the case of R1. Another reason could be the difference in specific irradiation in both reactors [23], 5.6 ± 1 (R1) and 48 ± 11 (R2), W gVSS−1, which could explain the lower PHA yield from the R1 reactor. This is likely due to the higher energy available for the PPB cultures in the smaller reactor due to higher volumetric light intensity. The excess of light causes an increase of the redox stress inside the cells due to overproduction of reductants like NADH in the electron transport chain, and it is one of the key factors for the accumulation of PHA by PPB [24]. However, the PHA production from both reactors remained stable at the end of each batch by maintaining up to 20 ± 1% in R1 and 31 ± 1% in R2 at an ORP of − 101 ± 14 mV in R1 and − 108 ± 6 mV in R2. The relatively high PHA production can be due to excess reductants from the organic carbon’s catabolism that enhance the PHA accumulation [1].
3.2 Biomass inactivation process
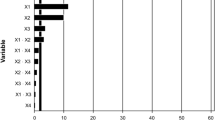
Once the higher PHA production was achieved in R1, the inactivation process was performed with different acids and compared with the control sample using formaldehyde. Figure 5 provides the detailed inactivation result of all three acids. The control sample treated with formaldehyde solution had a PHA fraction in biomass of control sample which amounted to 20.2%. However, the maximum PHA concentration was 33.4% by fixing the biomass with HNO3 at pH 2 for 4 h. The highest PHA of 30.7% and 32.2% was observed from HCl and HNO3, respectively, in inactivated samples at pH 1 for 8 h, whereas the maximum of 28.4% PHA was observed from H2SO4 in inactivated samples at pH 1 for 1 h. From the results, it is clear that, at pH 1, most of the acids worked effectively to inhibit the biomass PHA assimilation and resulted in higher PHA recovery. However, pH 3 also resulted in higher PHA recovery for the samples inactivated with HCl and HNO3. Thus, based on economic aspects, pH 3 can be considered an adequate acidity for fixing PPB biomass with HCl and HNO3. The results indicate that the PHA recovery was higher in the samples inactivated with acids than in the control sample inactivated with formaldehyde, which indicates that alternative inactivation with H2SO4, HNO3, and HCl was better than the traditional inactivation in terms of PHA recovery. The loss of the PHA assimilation and the less PPB consumption by acid inactivation were due to inhibition at low pH [25]. The low pH can destructure the cell wall by the acidic attack, causing general inhibition of the metabolism due to the PPB’s inability to perform active transport along the membranes. Another reason can be the disruption of the proton motive force or proton gradient, which may cause disability of the bacteria to produce electron transporters that are necessary to polymerize PHA and depolymerize PHA (phaZ, depolymerase enzyme) [26]. PHA synthesis and degradation can happen simultaneously [26]. Since inorganic acids disrupted cell metabolism, the polymerization and depolymerization of PHA stopped. Thus, the PHA was stabilized by the acidic treatment, likely due to metabolic inhibition. Additionally, inorganic acids do not degrade the accumulated PHA like alkali [13], and thus, higher PHA recovery can be achieved with acid inactivation.
The same samples were tested at day 0, again after one week (day 7), and after two weeks (day 14) to observe the stability of the PHA results achieved from alternative inactivation. Table 1 provides detailed data. The samples inactivated by HCl and HNO3 had relatively stable results even after day 14 compared to day 0. However, the stability test indicated that even though H2SO4 performed well for PHA inactivation, the results were not stable after day 7 and day 14 in samples inactivated at pH 3 for 8 h. Therefore, HCl and HNO3 were more effective inactivating agents for PHA. Alternatively, H2SO4 can also be used as an inactivating agent if stability is not an issue (e.g., in case the shipment of PHA-containing biomass is not necessary and PHA extraction can be performed onsite immediately after biomass inactivation). Also, pH 3 can be considered a suitable pH to fix PHA due to its economic benefits at a large scale due to lower acid requirements. The stability results also indicate that PHA does not degrade with time and remains stable for up to 2 weeks. On the other hand, the less hazardous could be considered to have fewer environmental hazards among the three inorganic acids. Thus, HNO3 can be considered an excellent inactivating agent as it can be economical for large-scale usage and does not have as much environmental impact as formaldehyde as an inactivating agent [14, 15], which causes environmental and health hazards. Consequently, the economic and environment-friendly inactivating agent can significantly reduce the PHA production costs, which is one of the major limitations to its industrialization [8].
3.3 Downstream processing and economic advantages
Recently, PHA production has gained enough attention in the context of industrial interest. However, large-scale PHA production is still costly and is one of the major limitations of bioplastic production. The PHA production costs until 2018 were 3.1€ kg−1, significantly higher than the production of fossil-based plastics, which costs 1.05–1.15 € kg−1 [2]. In industrial or large-scale PHA production, the major cost required is for microbial PHA polymer extraction from inside the cell [11]. The extraction process is a major cost factor besides feedstock cost for PHA production. Additionally, downstream processing has a large ecological footprint due to using chemicals, solvents, and energy [10]. In the past few years, several ongoing studies on PHA recovery aimed to reduce or substitute the use of chemicals and solvents to decrease the ecological footprint and maximize PHA yields [27]. However, since PHA recovery from PPB biomass requires stopping its metabolism, the recovery can be affected by changing biomass inactivation techniques.
PHA recovery from inside the cell of PPB biomass is generally performed after using solvents in the form of inactivating agents to stop PPB metabolism that consumes PHA. This is especially relevant when production and extraction processes are not performed in the same facilities, and the transport of biomass is necessary. Otherwise, synthesis and consumption of PHA can cooccur. Several strategies have been implemented at the production stage to reduce the inactivation process cost and decrease environmental hazards. However, the inactivation of PPB biomass to stop their metabolism and maximize PHA productivity is one of the major challenges which still has not been addressed. Mainly, formaldehyde is used as an inactivating agent for PPB biomass to stop their metabolism; however, it causes several environmental and health hazards. Moreover, the formaldehyde requirement can be high in large-scale PHA production, increasing production costs and environmental risk hazards. Therefore, finding other economic and environment-friendly alternatives for fixing PPB biomass is essential. Using inorganic acids such as H2SO4, HCl, and HNO3 as an inactivating agent for PPB can be a good alternative because of being economical and environmentally friendly. The cost associated with inorganic acids is estimated as much cheaper than the formaldehyde cost; e.g., considering the current market prices from a large chemical company (Merck) in a specific quality (ACS reagent) and the amounts needed to achieve the inactivation of the biomass in this study (expressed as € per gram of VSS), the relative application price for the inorganic acids and formaldehyde is 0.8, 0.43, 5.0, and 18.3 €/g for sulfuric (at pH 1), nitric and hydrochloric acids (at pH 3), and formaldehyde, respectively. In addition, inorganic acids do not degrade PHA like alkali, and higher PHA % can be achieved [13]. Additionally, the use of inorganic acids does not have any major environmental hazards; therefore, it would not have a large ecological footprint as the use of formaldehyde or other harmful solvents on a larger scale. Thereby, the use of inorganic acids can reduce operational costs significantly.
4 Conclusions
This study shows for the first time the possibility of using inorganic acids as an alternative inactivating agent for PPB biomass. The alternative inactivating agents were used to stop the PHA assimilation by PPB and to improve the PHA recovery potential. The study anticipates higher PHA recovery on a large scale. The PHA results indicated that with inorganic acid inactivation, the PHA recovery is higher than the use of formaldehyde. At pH 3, the results obtained from fixing with HCl and HNO3 were pretty stable; thus, it can be considered the best condition to fix PPB biomass. However, H2SO4 can also be used as an alternative except for 8 h at pH 3. Additionally, the stability of the results is even up to 2 weeks for all the inorganic acids except H2SO4 at pH 3. Using inorganic acids can benefit large-scale PHA production since it is more economical than other harmful solvents. It may also reduce environmental hazards associated with using formaldehyde as an inactivating agent.
Data availability
The data is available upon request.
References
Ventura M, Puyol D, Melero JA (2021) The synergy of catalysis and biotechnology as a tool to modulate the composition of biopolymers (polyhydroxyalkanoates) with lignocellulosic wastes. Catal Today. https://doi.org/10.1016/j.cattod.2021.09.032
Allegue LD, Ventura M, Melero JA, Puyol D (2021) Integrated sustainable process for polyhydroxyalkanoates production from lignocellulosic waste by purple phototrophic bacteria. GCB Bioenergy 13:862–875. https://doi.org/10.1111/gcbb.12807
George DM, Vincent AS, Mackey HR (2020) An overview of anoxygenic phototrophic bacteria and their applications in environmental biotechnology for sustainable resource recovery. Biotechnol Rep 28. https://doi.org/10.1016/j.btre.2020.e00563
Ishii-Hyakutake M, Mizuno S, Tsuge T (2018) Biosynthesis and characteristics of aromatic polyhydroxyalkanoates. Polymers (Basel) 10. https://doi.org/10.3390/polym10111267
Koller M, Mukherjee A (2020) Polyhydroxyalkanoates - linking properties, applications, and end-of-life options. Chem Biochem Eng Q 34:115–29. https://doi.org/10.15255/CABEQ.2020.1819
Vasiliadou IA, Berná A, Manchon C, Melero JA, Martinez F, Esteve-Nuñez A, et al (2018) Biological and bioelectrochemical systems for hydrogen production and carbon fixation using purple phototrophic bacteria. Front Energy Res 6. https://doi.org/10.3389/fenrg.2018.00107
Fra-Vázquez A, Santorio S, Palmeiro-Sánchez T, Val del Río Á, Mosquera-Corral A (2019) PHA accumulation of a mixed microbial culture co-exists with ammonia partial nitritation. Chem Eng J 360:1255–61
Fradinho J, Allegue LD, Ventura M, Melero JA, Reis MAM, Puyol D (2021) Up-scale challenges on biopolymer production from waste streams by Purple Phototrophic Bacteria mixed cultures: a critical review. Bioresour Technol 327. https://doi.org/10.1016/j.biortech.2021.124820.
Koller M, Mukherjee A (2022) A new wave of industrialization of PHA biopolyesters. Bioengineering 9. https://doi.org/10.3390/bioengineering9020074.
Koller M, Mukherjee A (2022) 6 Polyhydroxyalkanoates (PHAs)-production, properties, and biodegradation. https://doi.org/10.1002/9783527827589.ch6
Amara AA, Moawad H (2011) PHAC synthases and PHA depolymerases: the enzymes that produce and degrade plastic. Spec Issue Biotechnol 12. https://doi.org/10.31436/iiumej.v12i4.171
Allegue LD, Ventura M, Melero JA, Puyol D (2022) Unraveling PHA production from urban organic waste with purple phototrophic bacteria via organic overload. Renew Sust Energ Rev 166. https://doi.org/10.1016/j.rser.2022.112687
Burniol-Figols A, Skiadas I, v., Daugaard AE, Gavala HN, (2020) Polyhydroxyalkanoate (PHA) purification through dilute aqueous ammonia digestion at elevated temperatures. J Chem Technol Biotechnol 95:1519–32. https://doi.org/10.1002/jctb.6345
Mannina G, Presti D, Montiel-Jarillo G, Carrera J, Suárez-Ojeda ME (2020) Recovery of polyhydroxyalkanoates (PHAs) from wastewater: a review. Bioresour Technol 297. https://doi.org/10.1016/j.biortech.2019.122478.
Allegue LD, Puyol D, Melero JA (2020) Novel approach for the treatment of the organic fraction of municipal solid waste: coupling thermal hydrolysis with anaerobic digestion and photo-fermentation. Sci Total Environ 714. https://doi.org/10.1016/j.scitotenv.2020.136845.
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA).
Jurado-Marban VH, Tapia-Bustos MA, Gonzalez-Garcia RA, Salgado-Manjarrez E, García-Peña EI (2019) Hydrogen production by a mixed photoheterotrophic culture: correlation between gene expression analysis and physiological behavior. Int J Hydrogen Energy 44:641–651. https://doi.org/10.1016/j.ijhydene.2018.10.197
Monroy I, Buitrón G (2020) Production of polyhydroxybutyrate by pure and mixed cultures of purple non-sulfur bacteria: a review. J Biotechnol 317:39–47. https://doi.org/10.1016/j.jbiotec.2020.04.012
Fernández-Dacosta C, Posada JA, Kleerebezem R, Cuellar MC, Ramirez A (2015) Microbial community-based polyhydroxyalkanoates (PHAs) production from wastewater: techno-economic analysis and ex-ante environmental assessment. Bioresour Technol 185:368–377. https://doi.org/10.1016/j.biortech.2015.03.025
de las Heras I, Molina R, Segura Y, Hülsen T, Molina MC, Gonzalez-Benítez N, et al (2020) Contamination of N-poor wastewater with emerging pollutants does not affect the performance of purple phototrophic bacteria and the subsequent resource recovery potential. J Hazard Mater 385. https://doi.org/10.1016/j.jhazmat.2019.121617.
Fradinho JC, Oehmen A, Reis MAM (2013) Effect of dark/light periods on the polyhydroxyalkanoate production of a photosynthetic mixed culture. Bioresour Technol 148:474–479. https://doi.org/10.1016/j.biortech.2013.09.010
Almeida JR, Fradinho JC, Carvalho G, Oehmen A, Reis MAM (2022) Dynamics of microbial communities in phototrophic polyhydroxyalkanoate accumulating cultures. Microorganisms 351. https://doi.org/10.3390/microorganisms10020351
Fradinho JC, Oehmen A, Reis MAM (2019) Improving polyhydroxyalkanoates production in phototrophic mixed cultures by optimizing accumulator reactor operating conditions. Int J Biol Macromol 126:1085–1092. https://doi.org/10.1016/j.ijbiomac.2018.12.270
Bayon-Vicente G, Wattiez R, Leroy B (2020) Global proteomic analysis reveals high light intensity adaptation strategies and polyhydroxyalkanoate production in Rhodospirillum rubrum cultivated with acetate as carbon source. Front Microbiol 11:464. https://doi.org/10.3389/FMICB.2020.00464/BIBTEX
Almeida JR, Serrano E, Fernandez M, Fradinho JC, Oehmen A, Reis MAM (2021) Polyhydroxyalkanoates production from fermented domestic wastewater using phototrophic mixed cultures. Water Res 197. https://doi.org/10.1016/j.watres.2021.117101.
Yañez L, Conejeros R, Vergara-Fernández A, Scott F (2020) Beyond intracellular accumulation of polyhydroxyalkanoic acids and polymer secretion. Front Bioeng Biotechnol 248. https://doi.org/10.3389/fbioe.2020.00248
Koller M (2020) Established and advanced approaches for recovery of microbial polyhydroxyalkanoate (PHA) biopolyesters from surrounding microbial biomass. Eurobiotech J 4:113–126. https://doi.org/10.2478/ebtj-2020-0013
Acknowledgements
DP greatly appreciates the economic support the Spanish Ministry of Economy provided through the Ramon y Cajal Program.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work has received funding from the Bio-based Industries Joint Undertaking (JU) under the European Union’s Horizon 2020 research and innovation program under grant agreement no. 837998. The JU receives support from the European Union’s Horizon 2020 research and innovation program and the Bio-based Industries Consortium.
Author information
Authors and Affiliations
Contributions
Conceptualization: John A. Villamil, Daniel Puyol. Data curation: Pratiksha Srivastava, John A. Villamil, Denise Ramírez. Formal analysis: Pratiksha Srivastava, John A. Villamil, Daniel Puyol. Funding acquisition: Juan A. Melero, Daniel Puyol. Investigation: Pratiksha Srivastava, Denise Ramírez. Methodology: John A. Villamil, Daniel Puyol. Project administration: Juan A. Melero, Fernando Martínez, Daniel Puyol. Resources: Juan A. Melero, Fernando Martínez, Daniel Puyol. Software: Pratiksha Srivastava, John A. Villamil. Supervision: John A. Villamil, Daniel Puyol. Validation: John A. Villamil, Daniel Puyol. Visualization: Pratiksha Srivastava, John A. Villamil. Writing—original draft: Pratiksha Srivastava. Writing—review and editing: John A. Villamil, Juan A. Melero, Fernando Martínez, Daniel Puyol.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
Purple bacteria growth achieved 33% polyhydroxyalkanoate recovery at a large scale.
Higher polyhydroxyalkanoate recovery with inorganic acid fixation.
Inorganic fixation has stability in polyhydroxyalkanoate recovery from day 0 to day 14.
No polyhydroxyalkanoate degradation with inorganic acid fixation.
Reduced production costs at a large scale.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Srivastava, P., Villamil, J.A., Melero, J.A. et al. Using inorganic acids to stop purple phototrophic bacteria metabolism improves PHA recovery at a large scale. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03810-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03810-z