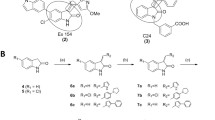
Abstract
Purpose
5′ adenosine monophosphate-activated kinase (AMPK) is an essential regulator of cellular energy homeostasis and has been associated with different pathologies, including cancer. Precisely defining the biological role of AMPK necessitates the availability of a potent and selective inhibitor.
Methods
High-throughput screening and chemical optimization were performed to identify a novel AMPK inhibitor. Cell proliferation and mechanistic assays, as well as gene expression analysis and chromatin immunoprecipitation were used to investigate the cellular impact as well as the crosstalk between lipid metabolism and androgen signaling in prostate cancer models. Also, fatty acid turnover was determined by examining lipid droplet formation.
Results
We identified BAY-3827 as a novel and potent AMPK inhibitor with additional activity against ribosomal 6 kinase (RSK) family members. It displays strong anti-proliferative effects in androgen-dependent prostate cancer cell lines. Analysis of genes involved in AMPK signaling revealed that the expression of those encoding 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR), fatty acid synthase (FASN) and 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 (PFKFB2), all of which are involved in lipid metabolism, was strongly upregulated by androgen in responsive models. Chromatin immunoprecipitation DNA-sequencing (ChIP-seq) analysis identified several androgen receptor (AR) binding peaks in the HMGCR and PFKFB2 genes. BAY-3827 strongly down-regulated the expression of lipase E (LIPE), cAMP-dependent protein kinase type II-beta regulatory subunit (PRKAR2B) and serine-threonine kinase AKT3 in responsive prostate cancer cell lines. Also, the expression of members of the carnitine palmitoyl-transferase 1 (CPT1) family was inhibited by BAY-3827, and this was paralleled by impaired lipid flux.
Conclusions
The availability of the potent inhibitor BAY-3827 will contribute to a better understanding of the role of AMPK signaling in cancer, especially in prostate cancer.





Similar content being viewed by others
Data availability
Complete ChIP-seq data are available at NCBI GEO (https://www.ncbi.nlm.nih.gov/geo/) under GSE148358.
Abbreviations
- ACC1:
-
acetyl-CoA carboxylase 1
- AMP:
-
adenosine monophosphate
- AMPK:
-
5′ adenosine monophosphate-activated kinase
- AR:
-
androgen receptor
- ATP:
-
adenosine triphosphate
- ATCC:
-
American Type Culture Collection
- CAMKK2:
-
calcium-calmodulin-dependent kinase 2
- ChIP-seq:
-
chromatin immunoprecipitation DNA-sequencing
- CPT1:
-
carnitine palmitoyl-transferase 1
- CRY1:
-
cryptochrome 1
- FASN:
-
fatty acid synthase
- DSMZ:
-
Deutsche Sammlung von Mikroorganismen und Zellkulturen
- GST:
-
glutathione-S-transferase
- HMGCR:
-
3-hydroxy-3-methyl-glutaryl-coenzyme A reductase
- HTRF:
-
homogeneous time-resolved fluorescence
- LIPE:
-
lipase E
- LKB1:
-
liver kinase B1
- PFKFB2:
-
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2
- PPARGC1B:
-
peroxisome proliferator-activated receptor gamma coactivator 1-beta
- PPP2CB:
-
serine/threonine-protein phosphatase 2A catalytic subunit beta isoform
- PRKAR2B:
-
cAMP-dependent protein kinase type II-beta regulatory subunit
- RSK:
-
ribosomal S6 kinase
- SREBF1:
-
sterol regulatory element-binding transcription factor 1
- TET2:
-
ten-eleven translocation 2
- TR-FRET:
-
time-resolved fluorescence resonance energy transfer
- ULK1:
-
autophagy activating kinase 1
References
F.A. Ross, C. MacKintosh, D.G. Hardie, AMP-activated protein kinase: A cellular energy sensor that comes in 12 flavours. FEBS J. 283, 2987–3001 (2016)
D.G. Hardie, B.E. Schaffer, A. Brunet, AMPK: An energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 26, 190–201 (2016)
D. Carling, AMPK signalling in health and disease. Curr. Opin. Cell Biol. 45, 31–37 (2017)
D.G. Hardie, Keeping the home fires burning: AMP-activated protein kinase. J. R. Soc. Interface 15, 20170774 (2018)
D.G. Hardie, S.C. Lin, AMP-activated protein kinase - not just an energy sensor. F1000Res 6, 1724 (2017)
L. Kullmann, M.P. Krahn, Controlling the master-upstream regulation of the tumor suppressor LKB1. Oncogene 37, 3045–3057 (2018)
M.R. Munday, C.J. Hemingway, The regulation of acetyl-CoA carboxylase--a potential target for the action of hypolipidemic agents. Adv. Enzym. Regul. 39, 205–234 (1999)
D. Wu, D. Hu, H. Chen, G. Shi, I.S. Fetahu, F. Wu, K. Rabidou, R. Fang, L. Tan, S. Xu, H. Liu, C. Argueta, L. Zhang, F. Mao, G. Yan, J. Chen, Z. Dong, R. Lv, Y. Xu, M. Wang, Y. Ye, S. Zhang, D. Duquette, S. Geng, C. Yin, C.G. Lian, G.F. Murphy, G.K. Adler, R. Garg, L. Lynch, P. Yang, Y. Li, F. Lan, J. Fan, Y. Shi, Y.G. Shi, Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 559, 637–641 (2018)
S. Umezawa, T. Higurashi, A. Nakajima, AMPK: Therapeutic target for diabetes and cancer prevention. Curr. Pharm. Des. 23, 3629–3644 (2017)
H.M. Haikala, J.M. Anttila, J. Klefstrom, MYC and AMPK-Save energy or die! Front. Cell. Dev. Biol. 5, 38 (2017)
S. Olivier, M. Foretz, B. Viollet, Promise and challenges for direct small molecule AMPK activators. Biochem. Pharmacol. 153, 147–158 (2018)
A.S. Khan, D.E. Frigo, A spatiotemporal hypothesis for the regulation, role, and targeting of AMPK in prostate cancer. Nat. Rev. Urol. 14, 164–180 (2017)
D.E. Frigo, M.K. Howe, B.M. Wittmann, A.M. Brunner, I. Cushman, Q. Wang, M. Brown, A.R. Means, D.P. McDonnell, CaM kinase kinase beta-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res. 71, 528–537 (2011)
P. Popovics, D.E. Frigo, A.V. Schally, F.G. Rick, Targeting the 5'-AMP-activated protein kinase and related metabolic pathways for the treatment of prostate cancer. Expert Opin. Ther. Targets 19, 617–632 (2015)
D. Awad, T.L. Pulliam, C. Lin, S.R. Wilkenfeld, D.E. Frigo, Delineation of the androgen-regulated signaling pathways in prostate cancer facilitates the development of novel therapeutic approaches. Curr. Opin. Pharmacol. 41, 1–11 (2018)
C.E. Massie, A. Lynch, A. Ramos-Montoya, J. Boren, R. Stark, L. Fazli, A. Warren, H. Scott, B. Madhu, N. Sharma, H. Bon, V. Zecchini, D.M. Smith, G.M. Denicola, N. Mathews, M. Osborne, J. Hadfield, S. Macarthur, B. Adryan, S.K. Lyons, K.M. Brindle, J. Griffiths, M.E. Gleave, P.S. Rennie, D.E. Neal, I.G. Mills, The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 30, 2719–2733 (2011)
L.G. Karacosta, B.A. Foster, G. Azabdaftari, D.M. Feliciano, A.M. Edelman, A regulatory feedback loop between Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) and the androgen receptor in prostate cancer progression. J. Biol. Chem. 287, 24832–24843 (2012)
J.B. Tennakoon, Y. Shi, J.J. Han, E. Tsouko, M.A. White, A.R. Burns, A. Zhang, X. Xia, O.R. Ilkayeva, L. Xin, M.M. Ittmann, F.G. Rick, A.V. Schally, D.E. Frigo, Androgens regulate prostate cancer cell growth via an AMPK-PGC-1alpha-mediated metabolic switch. Oncogene 33, 5251–5261 (2014)
Y. Choudhury, Z. Yang, I. Ahmad, C. Nixon, I.P. Salt, H.Y. Leung, AMP-activated protein kinase (AMPK) as a potential therapeutic target independent of PI3K/Akt signaling in prostate cancer. Oncoscience 1, 446–456 (2014)
R.R. Chhipa, Y. Wu, C. Ip, AMPK-mediated autophagy is a survival mechanism in androgen-dependent prostate cancer cells subjected to androgen deprivation and hypoxia. Cell. Signal. 23, 1466–1472 (2011)
S. Santha, N. Viswakarma, S. Das, A. Rana, B. Rana, Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-Troglitazone-induced apoptosis in prostate cancer cells involve AMP-activated protein kinase. J. Biol. Chem. 290, 21865–21875 (2015)
H.U. Park, S. Suy, M. Danner, V. Dailey, Y. Zhang, H. Li, D.R. Hyduke, B.T. Collins, G. Gagnon, B. Kallakury, D. Kumar, M.L. Brown, A. Fornace, A. Dritschilo, S.P. Collins, AMP-activated protein kinase promotes human prostate cancer cell growth and survival. Mol. Cancer Ther. 8, 733–741 (2009)
S. Jurmeister, A. Ramos-Montoya, D.E. Neal, L.G. Fryer, Transcriptomic analysis reveals inhibition of androgen receptor activity by AMPK in prostate cancer cells. Oncotarget 5, 3785–3799 (2014)
G. Zadra, C. Photopoulos, S. Tyekucheva, P. Heidari, Q.P. Weng, G. Fedele, H. Liu, N. Scaglia, C. Priolo, E. Sicinska, U. Mahmood, S. Signoretti, N. Birnberg, M. Loda, A novel direct activator of AMPK inhibits prostate cancer growth by blocking lipogenesis. EMBO Mol. Med. 6, 519–538 (2014)
L. Penfold, A. Woods, P. Muckett, A.Y. Nikitin, T.R. Kent, S. Zhang, R. Graham, A. Pollard, D. Carling, CAMKK2 promotes prostate cancer independently of AMPK via increased lipogenesis. Cancer Res. 78, 6747–6761 (2018)
C.H. Arrowsmith, J.E. Audia, C. Austin, J. Baell, J. Bennett, J. Blagg, C. Bountra, P.E. Brennan, P.J. Brown, M.E. Bunnage, C. Buser-Doepner, R.M. Campbell, A.J. Carter, P. Cohen, R.A. Copeland, B. Cravatt, J.L. Dahlin, D. Dhanak, A.M. Edwards, M. Frederiksen, S.V. Frye, N. Gray, C.E. Grimshaw, D. Hepworth, T. Howe, K.V. Huber, J. Jin, S. Knapp, J.D. Kotz, R.G. Kruger, D. Lowe, M.M. Mader, B. Marsden, A. Mueller-Fahrnow, S. Muller, R.C. O'Hagan, J.P. Overington, D.R. Owen, S.H. Rosenberg, B. Roth, R. Ross, M. Schapira, S.L. Schreiber, B. Shoichet, M. Sundstrom, G. Superti-Furga, J. Taunton, L. Toledo-Sherman, C. Walpole, M.A. Walters, T.M. Willson, P. Workman, R.N. Young, W.J. Zuercher, The promise and peril of chemical probes. Nat. Chem. Biol. 11, 536–541 (2015)
K. Kuramoto, H. Yamada, T. Shin, Y. Sawada, H. Azami, T. Yamada, T. Nagashima, K. Ohnuki, Development of a potent and orally active activator of adenosine monophosphate-activated protein kinase (AMPK), ASP4132, as a clinical candidate for the treatment of human cancer. Bioorg. Med. Chem. 28, 115307 (2020)
J. Bain, L. Plater, M. Elliott, N. Shpiro, C.J. Hastie, H. McLauchlan, I. Klevernic, J.S. Arthur, D.R. Alessi, P. Cohen, The selectivity of protein kinase inhibitors: A further update. Biochem. J. 408, 297–315 (2007)
L.G. Fryer, A. Parbu-Patel, D. Carling, Protein kinase inhibitors block the stimulation of the AMP-activated protein kinase by 5-amino-4-imidazolecarboxamide riboside. FEBS Lett. 531, 189–192 (2002)
J.W. Scott, S. Galic, K.L. Graham, R. Foitzik, N.X. Ling, T.A. Dite, S.M. Issa, C.G. Langendorf, Q.P. Weng, H.E. Thomas, T.W. Kay, N.C. Birnberg, G.R. Steinberg, B.E. Kemp, J.S. Oakhill, Inhibition of AMP-activated protein kinase at the allosteric drug-binding site promotes islet insulin release. Chem. Biol. 22, 705–711 (2015)
F.A. Ross, S.A. Hawley, F.R. Auciello, G.J. Gowans, A. Atrih, D.J. Lamont, D.G. Hardie, Mechanisms of paradoxical activation of AMPK by the kinase inhibitors SU6656 and sorafenib. Cell. Chem. Biol. 24, 813–824 e814 (2017)
T.A. Dite, C.G. Langendorf, A. Hoque, S. Galic, R.J. Rebello, A.J. Ovens, L.M. Lindqvist, K.R.W. Ngoei, N.X.Y. Ling, L. Furic, B.E. Kemp, J.W. Scott, J.S. Oakhill, AMP-activated protein kinase selectively inhibited by the type II inhibitor SBI-0206965. J. Biol. Chem. 293, 8874–8885 (2018)
D.F. Egan, M.G. Chun, M. Vamos, H. Zou, J. Rong, C.J. Miller, H.J. Lou, D. Raveendra-Panickar, C.C. Yang, D.J. Sheffler, P. Teriete, J.M. Asara, B.E. Turk, N.D. Cosford, R.J. Shaw, Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol. Cell 59, 285–297 (2015)
C.J. Matheson, K.A. Casalvieri, D.S. Backos, M. Minhajuddin, C.T. Jordan, P. Reigan, Substituted oxindol-3-ylidenes as AMP-activated protein kinase (AMPK) inhibitors. Eur. J. Med. Chem. 197, 112316 (2020)
V.K. Schulze, T. Heinrich, C. Christ, H. Briem, A.C. Faria Alvares de Lemos, B. Bader, S. Holton, U. Bömer, P. Lienau, L.P. Kuhnke, 4-(3-amino-6-fluoro-1H-indazol-5-yl)-1,2,6-trimethyl-1,4-dihydropyridine-3,5-dicarbonitrile compounds for treating hyperproliferative disorders. Patent application WO2019/185525 A1 (2019)
J. Barretina, G. Caponigro, N. Stransky, K. Venkatesan, A.A. Margolin, S. Kim, C.J. Wilson, J. Lehar, G.V. Kryukov, D. Sonkin, A. Reddy, M. Liu, L. Murray, M.F. Berger, J.E. Monahan, P. Morais, J. Meltzer, A. Korejwa, J. Jane-Valbuena, F.A. Mapa, J. Thibault, E. Bric-Furlong, P. Raman, A. Shipway, I.H. Engels, J. Cheng, G.K. Yu, J. Yu, P. Aspesi Jr., M. de Silva, K. Jagtap, M.D. Jones, L. Wang, C. Hatton, E. Palescandolo, S. Gupta, S. Mahan, C. Sougnez, R.C. Onofrio, T. Liefeld, L. MacConaill, W. Winckler, M. Reich, N. Li, J.P. Mesirov, S.B. Gabriel, G. Getz, K. Ardlie, V. Chan, V.E. Myer, B.L. Weber, J. Porter, M. Warmuth, P. Finan, J.L. Harris, M. Meyerson, T.R. Golub, M.P. Morrissey, W.R. Sellers, R. Schlegel, L.A. Garraway, The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012)
S.J. Baumgart, E. Nevedomskaya, R. Lesche, R. Newman, D. Mumberg, B. Haendler, Darolutamide antagonizes androgen signaling by blocking enhancer and super-enhancer activation. Mol. Oncol. 14, 2022–2039 (2020)
H. Li, R. Durbin, Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009)
H. Wang, E. Wei, A.D. Quiroga, X. Sun, N. Touret, R. Lehner, Altered lipid droplet dynamics in hepatocytes lacking triacylglycerol hydrolase expression. Mol. Biol. Cell 21, 1991–2000 (2010)
D. Vara-Ciruelos, F.M. Russell, D.G. Hardie, The strange case of AMPK and cancer: Dr Jekyll or Mr Hyde? Open Biol. 9, 190099 (2019)
Y. Kong, L. Cheng, F. Mao, Z. Zhang, Y. Zhang, E. Farah, J. Bosler, Y. Bai, N. Ahmad, S. Kuang, L. Li, X. Liu, Inhibition of cholesterol biosynthesis overcomes enzalutamide resistance in castration-resistant prostate cancer (CRPC). J. Biol. Chem. 293, 14328–14341 (2018)
C. Liu, W. Lou, Y. Zhu, J.C. Yang, N. Nadiminty, N.W. Gaikwad, C.P. Evans, A.C. Gao, Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res. 75, 1413–1422 (2015)
J.S. Moon, W.J. Jin, J.H. Kwak, H.J. Kim, M.J. Yun, J.W. Kim, S.W. Park, K.S. Kim, Androgen stimulates glycolysis for de novo lipid synthesis by increasing the activities of hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2 in prostate cancer cells. Biochem. J. 433, 225–233 (2011)
J.A. Locke, E.S. Guns, M.L. Lehman, S. Ettinger, A. Zoubeidi, A. Lubik, K. Margiotti, L. Fazli, H. Adomat, K.M. Wasan, M.E. Gleave, C.C. Nelson, Arachidonic acid activation of intratumoral steroid synthesis during prostate cancer progression to castration resistance. Prostate 70, 239–251 (2010)
S. Balaban, R.F. Shearer, L.S. Lee, M. van Geldermalsen, M. Schreuder, H.C. Shtein, R. Cairns, K.C. Thomas, D.J. Fazakerley, T. Grewal, J. Holst, D.N. Saunders, A.J. Hoy, Adipocyte lipolysis links obesity to breast cancer growth: Adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 5, 1 (2017)
T.W. Flaig, M. Salzmann-Sullivan, L.J. Su, Z. Zhang, M. Joshi, M.A. Gijon, J. Kim, J.J. Arcaroli, A. Van Bokhoven, M.S. Lucia, F.G. La Rosa, I.R. Schlaepfer, Lipid catabolism inhibition sensitizes prostate cancer cells to antiandrogen blockade. Oncotarget 8, 56051–56065 (2017)
M. Joshi, G.E. Stoykova, M. Salzmann-Sullivan, M. Dzieciatkowska, L.N. Liebman, G. Deep, I.R. Schlaepfer, CPT1A supports castration-resistant prostate cancer in androgen-deprived conditions. Cells 8, 1115 (2019)
H.P. Lin, C.Y. Lin, C. Huo, Y.J. Jan, J.C. Tseng, S.S. Jiang, Y.Y. Kuo, S.C. Chen, C.T. Wang, T.M. Chan, J.Y. Liou, J. Wang, W.S. Chang, C.H. Chang, H.J. Kung, C.P. Chuu, AKT3 promotes prostate cancer proliferation cells through regulation of Akt, B-Raf, and TSC1/TSC2. Oncotarget 6, 27097–27112 (2015)
J. Sha, W. Xue, B. Dong, J. Pan, X. Wu, D. Li, D. Liu, Y. Huang, PRKAR2B plays an oncogenic role in the castration-resistant prostate cancer. Oncotarget 8, 6114–6129 (2017)
P. Gonzalez-Menendez, D. Hevia, J.C. Mayo, R.M. Sainz, The dark side of glucose transporters in prostate cancer: Are they a new feature to characterize carcinomas? Int. J. Cancer 142, 2414–2424 (2018)
Y. Liu, L.S. Zuckier, N.V. Ghesani, Dominant uptake of fatty acid over glucose by prostate cells: A potential new diagnostic and therapeutic approach. Anticancer Res. 30, 369–374 (2010)
M. Kaarbo, T.I. Klokk, F. Saatcioglu, Androgen signaling and its interactions with other signaling pathways in prostate cancer. Bioessays 29, 1227–1238 (2007)
V. Cucchiara, J.C. Yang, V. Mirone, A.C. Gao, M.G. Rosenfeld, C.P. Evans, Epigenomic regulation of androgen receptor signaling: Potential role in prostate cancer therapy. Cancers (Basel) 9(9) (2017)
E. Eidelman, J. Twum-Ampofo, J. Ansari, M.M. Siddiqui, The metabolic phenotype of prostate cancer. Front. Oncol. 7, 131 (2017)
L. Galbraith, H.Y. Leung, I. Ahmad, Lipid pathway deregulation in advanced prostate cancer. Pharmacol. Res. 131, 177–184 (2018)
K.D. Tousignant, A. Rockstroh, A. Taherian Fard, M.L. Lehman, C. Wang, S.J. McPherson, L.K. Philp, N. Bartonicek, M.E. Dinger, C.C. Nelson, M.C. Sadowski, Lipid uptake is an androgen-enhanced lipid supply pathway associated with prostate cancer disease progression and bone metastasis. Mol. Cancer Res. 17, 1166–1179 (2019)
X. Wu, G. Daniels, P. Lee, M.E. Monaco, Lipid metabolism in prostate cancer. Am. J. Clin. Exp. Urol. 2, 111–120 (2014)
F. Giunchi, M. Fiorentino, M. Loda, The metabolic landscape of prostate cancer. Eur. Urol. Oncol. 2, 28–36 (2019)
G.E. Stoykova, I.R. Schlaepfer, Lipid metabolism and endocrine resistance in prostate cancer, and new opportunities for therapy. Int. J. Mol. Sci. 20, 2626 (2019)
C.Y. Mah, Z.D. Nassar, J.V. Swinnen, L.M. Butler, Lipogenic effects of androgen signaling in normal and malignant prostate. Asian J. Urol. 7, 258–270 (2020)
G. Yu, Y.C. Lee, C.J. Cheng, C.F. Wu, J.H. Song, G.E. Gallick, L.Y. Yu-Lee, J. Kuang, S.H. Lin, RSK promotes prostate cancer progression in bone through ING3, CKAP2, and PTK6-mediated cell survival. Mol. Cancer Res. 13, 348–357 (2015)
D.E. Clark, T.M. Errington, J.A. Smith, H.F. Frierson Jr., M.J. Weber, D.A. Lannigan, The serine/threonine protein kinase, p90 ribosomal S6 kinase, is an important regulator of prostate cancer cell proliferation. Cancer Res. 65, 3108–3116 (2005)
M. Shiota, M. Itsumi, A. Yokomizo, A. Takeuchi, K. Imada, E. Kashiwagi, J. Inokuchi, K. Tatsugami, T. Uchiumi, S. Naito, Targeting ribosomal S6 kinases/Y-box binding protein-1 signaling improves cellular sensitivity to taxane in prostate cancer. Prostate 74, 829–838 (2014)
M. Roffe, F.C. Lupinacci, L.C. Soares, G.N. Hajj, V.R. Martins, Two widely used RSK inhibitors, BI-D1870 and SL0101, alter mTORC1 signaling in a RSK-independent manner. Cell. Signal. 27, 1630–1642 (2015)
Acknowledgments
We thank the project team for continuous support. The help of Maria Quanz, Daniel Seifert, Fanny Knoth, Hagen Muckwar, Enrico Spelling, Carolin Pohle, Guido Piechowiak, Sebastian Schulze, Janine Fischer, Vivien Raschke, Pia Stollberg and Daniel Wolleh is gratefully acknowledged. We are indebted to Martin Eilers (University of Würzburg, Germany) for scientific advice. Support with chemical syntheses by Pharmaron is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Clara Lemos, Volker Schulze and Bernard Haendler contributed to the study conception and design. Medicinal chemistry and computational chemistry: Volker Schulze, Tobias Heinrich, Julien Lefranc, Hans Briem, Lara Kuhnke and Clara Christ were in charge of the medicinal chemistry and computational chemistry part. Clara Lemos, Simon Baumgart, Benjamin Bader, Simon Holton, Ulf Bömer, Philip Lienau and Bernard Haendler were involved in the acquisition of pharmacology data. Clara Lemos, Volker Schulze, Simon Baumgart, Ekaterina Nevedomskaya, Benjamin Bader, Clara Christ, Ulf Bömer and Bernard Haendler were involved in the analysis and interpretation of data (i.e., statistical analysis, biostatistics, computational analysis). Franz von Nussbaum, Carl Nising, Marcus Bauser, Andrea Hägebarth and Dominik Mumberg supervised the study. The first draft of the manuscript was written by Bernard Haendler and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors are/were employees and/or shareholders of Bayer AG.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PPTX 6797 kb)
Rights and permissions
About this article
Cite this article
Lemos, C., Schulze, V.K., Baumgart, S.J. et al. The potent AMPK inhibitor BAY-3827 shows strong efficacy in androgen-dependent prostate cancer models. Cell Oncol. 44, 581–594 (2021). https://doi.org/10.1007/s13402-020-00584-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00584-8