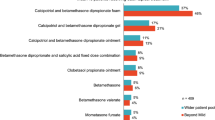
Abstract
Introduction
There are clear treatment options for mild psoriasis where topical therapies are the mainstay, and for severe psoriasis where systemic therapy (biologic or non-biologic) is necessary. However, there is less clarity in the ‘grey zone’ of patients in the moderate or so-called ‘beyond-mild’ segment. There are frequent delays to the initiation, discontinuation, switching and dose change in treatment, and many patients fail to continue treatment because of concerns about safety or lack of efficacy. Treatment with topical therapies, such as calcipotriol and betamethasone dipropionate (Cal/BD) combinations, may be suitable for these patients.
Method
These consensus recommendations on the use of topical therapies including Cal/BD foam for beyond-mild psoriasis originated from a modified Delphi process of European clinical experts. In the process, the experts iteratively refined a series of draft statements, which had to receive ≥ 80% approval to be incorporated into the consensus.
Results
The experts identified three main themes: Cal/BD foam as monotherapy, as an add-on to non-biologic systemic therapies and as an add-on to systemic biologics. The consensus emphasises disease factors and patient preference in treatment choice, summarises the evidence base for Cal/BD foam monotherapy for flare treatment as well as long-term management, and identifies the potential for improved treatment outcomes, such as reduced time to onset of action and reduced systemic dose to minimise side effects for add-on Cal/BD therapy to non-biologic systemics. The recommendations regarding add-on Cal/BD foam to biologics are similar to those for non-biologic systemic therapies, but also include suggestions for patients on biologics who are late responders. As clinical choices of Cal/BD combination vary, we have here often used ‘Cal/BD’ without reference to any particular formulation.
Conclusions
These recommendations aim to give practical guidance to those treating patients with beyond-mild psoriasis, to support patients’ use of topical preparations and to optimise treatment outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Determining optimal treatment for moderate plaque psoriasis can be challenging as there exists a grey area between the mild and severe ends of the spectrum, hereby referred to as ‘beyond-mild’, where optimal patient management is uncertain. |
Following growing evidence for the use of calcipotriol and betamethasone dipropionate (Cal/BD) foam for beyond-mild psoriasis, we conducted a modified Delphi review to identify key themes and recommendations for treatment based on currently available data and expert clinical experience and opinion. |
What was learned from the study? |
Three key themes regarding the use of Cal/BD foam in the beyond-mild psoriasis patient were identified. These were the use of Cal/BD foam as: (1) monotherapy, (2) add-on to non-biologic systemic therapies and (3) add-on to biologics. |
Across these three themes, the authors make 14 key recommendations for the use of CAL/BD foam in adult patients (summarised in Tables 1,2,3). |
These recommendations are intended to help provide healthcare professionals (HCPs) with guidance to support their use of the topical medication Cal/BD foam, as monotherapy or as add-on treatment with non-biologic or biologic systemic therapy for beyond-mild psoriasis, and ultimately to optimise treatment outcomes for these patients. |
Introduction
Psoriasis is a chronic, inflammatory, immune-mediated disease which primarily affects the skin and joints, and occurs in 2–4% of the Western population [1]. In addition to bothersome physical symptoms, psoriasis is often associated with significant psychosocial burden as a result of social stigmatisation, and difficulties with body image and self-esteem are experienced by many patients. Psoriasis may, therefore, have a profound impact on the patient’s quality of life (QoL) [2, 3].
Although there are clear treatment options for mild and severe psoriasis, there exists a ‘grey zone’ between these two types. The American Academy of Dermatology classifies moderate psoriasis as that which affects from at least 5% to less than 10% of body surface area (BSA), while European guidelines classify moderate-to-severe psoriasis as that which affects more than 10% of BSA [4, 5]. Topical therapies are the mainstay for mild-to-moderate psoriasis [6], but can also be used as an add-on to systemic therapy (non-biologic or biologic) [5, 7]. Topical steroids are effective and inexpensive but have limitations in terms of locations, such as the face and intertriginous areas, where they are not recommended (except for very short-term use) owing to local side effects [8]. In other areas, topical steroids may be applied for longer periods but are recommended for use beyond 12 weeks only under careful medical supervision [9]. Multiple topical agents are used in psoriasis to supplement and reduce over-reliance on topical steroids. These agents include vitamin D analogues, retinoids such as tazarotene and off-label use of calcineurin inhibitors [8, 9]. Other topical treatments include salicylic acid, dithranol and coal tar preparations [9].
A common treatment pathway for mild forms of plaque psoriasis is daily treatment either with a topical corticosteroid or a fixed topical combination of calcipotriol and betamethasone dipropionate (Cal/BD) with evaluation of response in 2–8 weeks. If there is a response to treatment, frequency of treatment can be reduced, for example to twice weekly. If not, UV or systemic therapy at an expert centre may be needed [8]. For patients with more severe psoriasis, non-biologic systemic treatments are sometimes recommended as first-line therapy (e.g. methotrexate, cyclosporin and acitretin). Biologics are also recommended for these patients when they fail to respond or have contraindications to/side effects from non-biologic systemics [10,11,12,13].
Patients in the ‘grey zone’ between mild and severe may be eligible for topical or systemic therapy, or a combination of both. Following a review of the academic literature, three psoriasis specialists, in collaboration with LEO Pharma and a market research company (Cello Health Insight, London, UK), developed the concept of ‘beyond-mild’ psoriasis to describe this population [14]. The literature review selected studies with the following characteristics: patients with moderate-to-severe disease; N > 50 patients; treatment either available or with potential to be licensed by the European Medicines Agency. More weight was given to studies with an active comparator (not placebo/vehicle), and informative severity measures and outcomes [14].
While attention in recent years has focused on biologics for the treatment of moderate-to-severe psoriasis, advances have also been made in the development of topical agents [15], such as fixed-dose combinations of Cal/BD in gel, ointment and foam formulations, and of halobetasol propionate and tazarotene. In addition, the use of steroid-sparing agents can reduce the risk of corticosteroid-related adverse effects [9]. The recent PSO-LONG phase III trial demonstrated that long-term proactive management over 52 weeks with fixed-dose Cal/BD foam was superior in (1) prolonging time to first relapse, (2) reducing number of relapses and (3) increasing days in remission, compared with vehicle foam, in adults with plaque psoriasis, with a favourable safety profile [16].
Additionally, there is growing clinical evidence to support the use of Cal/BD formulations for the treatment of patients with moderate-to-severe psoriasis, both as monotherapy (particularly the foam) [17] and as add-on therapy to non-biologic systemic [18] or biologic treatments [19]. In addition, real-world data demonstrate the use of Cal/BD foam for patients with beyond-mild psoriasis as monotherapy or as part of a multi-therapy strategy with other topical or systemic agents [12].
Based on the data available for Cal/BD formulations, and to arrive at a clinical consensus for the use of Cal/BD foam in beyond-mild psoriasis, we conducted a modified Delphi review based on currently available data and expert clinical experience and opinion. While more detailed data are available for the Cal/BD foam formulation than for other topical treatments, recommendations may be applicable to other topical treatments in the context of beyond-mild psoriasis.
Methods
The Consensus Process
An advisory group of nine European-based expert dermatologists from five countries (France, Germany, Italy, Spain and UK) met in September 2019. Advisors had extensive clinical experience in treating mild-to-severe psoriasis. Initial discussions focused on the identification of clinical care gaps or themes in relation to the treatment of beyond-mild psoriasis, where the development of key recommendations for treatment of beyond-mild psoriasis could be considered valuable. The advisors then evaluated each clinical theme and generated supporting statements to provide clinical guidance. Using a modified Delphi methodology [20], draft statements were then reviewed and refined, if necessary, based on clinical value and evidence.
The advisors voted on the draft statements. Consensus was defined as ≥ 80% agreement with the summary statement. During the voting process, each advisor assigned an ‘agreement score’ from 1 to 5 to each statement, where 1 denoted their strong disagreement and 5 denoted strong agreement. Individual scores were then collated and assigned to one of three groups: 1–2, 3 and 4–5. A strong level of agreement to a given statement was achieved if ≥ 80% of advisors scored within the 4–5 range. Statements for which an agreement was not achieved were discussed, revised and voted on again. If agreement was not achieved after this second vote, a lack of agreement was recorded. Some slight amendment of statements has been made during preparation of this publication to maintain consistency. This paper is formed of the opinions of the authors themselves and contains no research or study elements that would require ethics committee approval.
Results
Three key themes regarding the use of Cal/BD foam in the beyond-mild psoriasis patient were identified. These were the use of Cal/BD foam as: (1) monotherapy, (2) add-on to non-biologic systemic therapies and (3) add-on to biologics.
Cal/BD Foam as Monotherapy
The advisors provided four key recommendations on use of Cal/BD foam as monotherapy (Table 1). These recommendations are listed below.
Recommendation 1A: Use of Cal/BD Foam as Monotherapy Should Be Guided by HCPs’ Consideration of Disease Factors, Including PASI > 10 or BSA > 10% or DLQI > 10
Variation in the measurement of the severity of psoriasis is reflected in a range of definitions in current guidelines [2, 8, 9, 21]. In practice, healthcare professionals (HCPs) should consider a number of parameters when assessing psoriasis severity, as well as traditional tools that assess the objective characteristics of the disease, such as BSA and the Psoriasis Area and Severity Index (PASI). Psoriasis severity can also be underestimated if prior treatment failure history and/or relevant impact of psoriasis on QoL is not taken into account [22]. The location of lesions and a measurement of QoL, such as the Dermatology Life Quality Index (DLQI), are important for accurate assessment of psoriasis severity [22].
Some guidelines already consider QoL in psoriasis management. The European consensus on treatment goals for moderate-to-severe psoriasis follows the ‘rule of tens’ – BSA > 10, PASI > 10 or DLQI > 10 – in its definition of moderate-to-severe psoriasis [22]. The British and Canadian guidelines acknowledge the importance of areas of involvement and psychosocial impact, as criteria for severity classification and treatment decisions [10, 23]. While Cal/BD foam is an option for those with beyond-mild psoriasis, it is important to note that topical monotherapy may not be appropriate in patients with extensive lesions. Cal/BD foam is not indicated for use in patients with BSA > 30%, nor on the face or genitalia [24].
Recommendation 1B: Cal/BD Foam, Given as Monotherapy, Is Safe and Effective up to 4 weeks for Patients with Beyond-Mild Psoriasis (as Supported by RCTs, Real-World Evidence and Guidelines)
Current guidelines recommend the use of topical monotherapy as first-line therapy in localised disease, and advise that improvement in symptoms should be expected within 4 weeks of initiating topical therapy [7, 25]. Clinical evidence supports the use of Cal/BD foam as monotherapy in patients with more severe disease/moderate-to-severe psoriasis, due to its efficacy, rapid onset of action and favourable safety profile [14, 17, 26,27,28]. For example, in a subgroup analysis of the PSO-ABLE study in patients with moderate-to-severe disease, a higher proportion of patients achieved PASI 75 with Cal/BD foam compared with gel at weeks 4, 8 and 12, and also had superior DLQI scores [17].
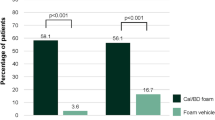
A real-world, prospective, observational study assessed the efficacy and safety of Cal/BD foam in 410 patients with mild-to-severe plaque psoriasis in daily clinical practice conditions. After 4 weeks of treatment, 43% of patients with severe psoriasis (Investigator’s Global Assessment (IGA) score = 4) were clear/almost clear of lesions and had improvements in IGA [16].
Recommendation 1C: Cal/BD Foam May Be Used to Bridge the Time to Starting Subsequent Systemic Treatment
Dermatologists may use topical treatments to ‘bridge’ the time to a patient first starting systemic therapies [29, 30]. Topical treatments may also be used as a bridge to cover a treatment gap or to control disease flare when, in some healthcare systems, formal approval is required from payers before systemic therapy is initiated or changed [31]. Real-world evidence (RWE) demonstrates that specialists may use topical agents to bridge the waiting time to systemic treatment with a non-biologic (64% of HCPs) or a biologic (63%) [14].
Recommendation 1D: Use of Cal/BD Foam as Monotherapy Should Be Guided by the Patient, Considering Their Preference for a Topical Agent Over a Systemic Therapy, Goals and Expectations for Treatment, and Desire for an Easier-to-Use Formulation
Patient preference is an important consideration when choosing a treatment [32]. For example, a patient may be reluctant to start systemic treatment and wish to see if treatment with topicals alone can resolve their disease. It is important to note, however, that when systemic treatment is needed it should not be delayed.
A shared decision-making process involving patient preference and clinician judgement may enhance outcomes, such as treatment satisfaction and adherence (a key factor for treatment efficacy) [32]. Suboptimal adherence is often the reason why real-life outcomes fail to reflect outcomes seen in clinical trials [33].
Cal/BD Foam as an Add-on to Non-biologic Systemic Therapies
The advisors provided six key recommendations on use of Cal/BD foam in combination with non-biologic systemic therapies. Four of the recommendations relate to the use of the foam in patients being initiated on non-biologic systemics, and two concern its use in patients already receiving non-biologic systemics (Table 2). These recommendations are listed below.
Recommendation 2A: Consider Cal/BD Foam as an Add-on When Starting a Non-biologic to Enhance Treatment Outcome and Time of Onset of Response
Many patients with psoriasis receiving systemic agents, such as methotrexate, do not experience an optimal response to treatment; some studies suggest only about 40% of patients achieve PASI 75 [34, 35]. In addition, some systemic treatments can have a slow onset of action, taking months to achieve maximum therapeutic response [22]. For patients initiated on a slower acting non-biologic, starting a topical therapy at the same time may achieve a quick and effective response when required. For example, Kircik found that adding Cal/BD foam to apremilast at the time of initiation in patients with moderate psoriasis improved the speed of onset and efficacy of overall treatment, as well as patient DLQI scores [18].
Recommendation 2B: Consider Combining Cal/BD Foam with a Non-biologic as an Add-on to Improve Treatment Outcomes in Patients Who Are Late Responders
Certain patients may experience a delayed response to systemic therapies (excluding cyclosporin) [36]. In these cases, add-on topical therapy should be offered to optimise treatment outcomes, as this may avoid the need to switch to another systemic agent [7, 37].
Recommendation 2C: For Responder Patients Experiencing Loss of Efficacy on a Non-biologic Therapy, Treatment May Be Optimised by the Addition of Cal/BD Foam
The ‘drug survival’ (duration of adherence) of non-biologics can be reduced in some patients. These patients may be candidates for add-on topical treatments [19]. The Swiss Dermatology Network for Targeted Therapies, a national psoriasis registry of patients with moderate-to-severe psoriasis treated with either a non-biologic or biologic, found that drug survival for non-biologic systemic treatments, including methotrexate, was 19.2 months [38]. Cal/BD foam may be a useful option in cases when the efficacy of the current non-biologic treatment has decreased over time.
Recommendation 2D: In Responder Patients Not Satisfied with Non-biologic Treatment (Assessed Using e.g. PASI, QoL and HADS), Addition of Cal/BD Foam May Be Considered
Some responder patients may be dissatisfied with their treatment despite achieving full or partial clinical success (e.g. PASI 75 or PASI 50–75, respectively). Suboptimal patient satisfaction with systemic therapies has been reported [39]. There is a need for more patient-centred assessments (e.g. the DLQI and Hospital Anxiety and Depression Scale (HADS) questionnaires) in a ‘treat-to-target’ approach to help better gauge patient satisfaction with treatment [40,41,42]. For patients not satisfied with their treatment outcome, the use of Cal/BD foam as an add-on to their non-biologic treatment may be an option.
Recommendation 2E: Consider Combining Cal/BD Foam and a Non-biologic Systemic Therapy, as it May Allow a Systemic Dose Reduction or Minimise Side Effects
If topicals increase the efficacy of a systemic non-biologic therapy, a lower dose of that systemic may achieve comparable clinical response to monotherapy. This may lower the risk of adverse events. Such use of topicals is supported in a review describing the combination of topicals with non-biologics. Adding a topical to methotrexate, phototherapy, acitretin or cyclosporin increased the overall/combined treatment efficacy and enabled use of a lower dose of the non-biologic systemic [19].
Recommendation 2F: Consider Combining Cal/BD Foam and a Non-Biologic Systemic Therapy to Control Residual Disease
Patients with psoriasis with resistant lesions and/or residual disease may experience reduced health-related QoL (HRQoL). Such patients may respond after addition of topical agents to conventional systemic treatments [5], and may also benefit from their use in managing isolated, difficult-to-treat areas [4, 7].
Topical treatments may also be added to non-biologic systemics for the control of psoriasis exacerbations. Psoriasis activity fluctuates over time, and topical therapies can be used at the first sign of increased activity to prevent disease escalation. Patients can, therefore, use topicals to minimise flares and maximise control of the condition [43].
Cal/BD Foam as an Add-on to Biologics
The experts provided four recommendations on use of Cal/BD foam in combination with biologics. Two of the recommendations are about Cal/BD foam in patients initiated on biologics, while the remaining two concern use in patients already receiving biologics (Table 3). These recommendations are listed below.
Recommendation 3A: Consider Using Cal/BD Foam as an Add-on When Starting a Biologic to Enhance Treatment Outcomes and Time to Onset of Response
There may be situations where use of a topical agent as an add-on is beneficial for patients with extensive psoriasis who are receiving biologics [5]. When a biologic is newly started, addition of a topical agent may increase the overall efficacy of treatment (and overall disease improvement) by treating residual disease or potentially reducing the time to onset of action of a slower-acting biologic [5, 44].
In clinical practice, the effectiveness of biologics can be lower than the efficacy reported in clinical trials [45]. Based on Physician Global Assessment (PGA), the efficacy of adalimumab was 73% in a randomised controlled trial (RCT), but 48% in a real-world study [45]. Clinical trial findings support the use of add-on topical therapy with biologics to enhance treatment effectiveness. A phase IIIb, multicentre RCT in patients with moderate-to-severe plaque psoriasis demonstrated that short-term use of topical clobetasol propionate (0.05%) with etanercept provided additional clinical benefit at week 12 in terms of PASI 75, PGA ‘clear/almost clear’ and BSA, compared with etanercept alone [46].
Some biologics can have a time to onset of action of up to 25 weeks [47]. Adding a topical treatment to a slow-acting biologic at treatment initiation may help achieve the quickest and greatest therapeutic response. The BELIEVE study reported that the addition of Cal/BD foam to adalimumab in patients with moderate-to-severe psoriasis resulted in more rapid and efficacious responses in the first 4 weeks versus patients receiving adalimumab alone (p = 0.021) [48].
Combination of a topical treatment with systemic therapy is a frequently used strategy for moderate-to-severe psoriasis (BSA > 10%) according to a recent survey of international dermatologists [49].
Recommendation 3B: Consider Combining Cal/BD Foam as an Add-on When Starting a Biologic to Improve Treatment Outcomes in Late-Responder Patients Who May Not Immediately Respond to a Biologic
The response to biologics can be delayed [50]. An initial lack of response to biologics may impact a patient’s treatment satisfaction and adherence, and may prompt a switch away from their current biologic. The use of Cal/BD foam in these circumstances may improve outcomes and prevent the need for switching.
Recommendation 3C: For Responder Patients Experiencing Reduced Efficacy on a Biologic, Treatment May Be Optimised by the Addition of Cal/BD Foam
The ‘drug survival’ (duration of adherence) of certain biologics can be reduced because of factors such as anti-drug antibodies [51,52,53]. A meta-analysis of 37 studies, which pooled drug survival of biologics for treatment of psoriasis in 32,631 patients, found that drug survival for all four of the biologics studied was reduced from year 1 to year 4 [52, 53]. These results are consistent with findings from the Danish DERMBIO registry, which reported that the efficacy of biologics diminishes over time and that this reduced efficacy is responsible for the majority of patient discontinuations [53].
Finally, add-on topical treatment with biologics may also be used to enhance treatment outcomes for patients experiencing secondary loss of efficacy or a seasonal fluctuation/flare/exacerbation of their disease while receiving a biologic [54].
Recommendation 3D: In Responder Patients Not Satisfied with Their Biologic Treatment (Assessed Using e.g. PASI, QoL, HADS), Addition of Cal/BD Foam May Be Considered
Achieving patient satisfaction with systemic therapies can be challenging, and lack of patient satisfaction with biologic treatment represents a significant problem. It can adversely affect patient adherence, patient preferences and HRQoL [55].
Evidence from BioCAPTURE, a daily-practice registry consisting of patients with psoriasis treated with biologics, suggests that poor satisfaction, measured by the Treatment Satisfaction Questionnaire for Medication (TSQM) among female patients, may contribute to their earlier discontinuation of biologic treatment versus male patients [56]. A prospective, open-label study evaluated Cal/BD foam efficacy in patients who were already receiving biologics, but in whom treatment responses were inadequate. Add-on therapy with Cal/BD foam was found to improve both patient HRQoL and treatment satisfaction (as measured by DLQI and TSQM-9 at weeks 4 and 16) [57].
Discussion
Treatments for psoriasis are constantly evolving, and up-to-date recommendations on therapy are therefore imperative. This is particularly the case for the beyond-mild population, for whom there is a lack of clarity on treatment options. We identified situations in which the beyond-mild psoriasis population may benefit from the use of a topical treatment, either alone or in addition to an existing systemic therapy. Topical treatment with Cal/BD combinations including Cal/BD foam is an add-on option to non-biologic and biologic therapy to improve outcomes, reduce the time to onset of action, and reduce the dose of systemic therapy to minimise side effects; it may also help enhance outcomes in patients with a delayed response to biologic therapy. These recommendations do not address circumstances in which treatments may be restricted or contraindicated.
The side effects associated with a treatment are a key factor in determining its successful use, and patients may avoid a treatment because of adverse reactions. Additionally, some systemic treatments are contraindicated in patients with comorbidities. For example, oral methotrexate should be given ‘with great caution if at all’ when patients have hepatic disease, and it is associated with pulmonary fibrosis in rare circumstances [58]. Pregnancy or drug interactions may also limit treatment options [59]. Contraindications specific to biologics may include situations in which requirements for laboratory monitoring during treatment or refrigerated storage are not feasible [60], while cost may be an additional barrier [61].
Topical treatments have fewer associated side effects and contraindications than systemic therapies, but concerns remain over long-term patient adherence [62]. Ease of use, time to achieve satisfactory efficacy, cost and patient acceptability may be barriers to adherence, and patients may prefer some formulations to others [63]. The area to be treated is also a consideration: patients may prefer to use and be more adherent to shampoo products for scalp psoriasis [9]. Other limitations of topicals include the occurrence of ‘topical fatigue’ or tachyphylaxis and restrictions in the total BSA that can be treated [24]. However, novel topical treatments may have improved acceptability to patients over traditional creams or ointments because of enhanced ease of application or absence of odour [29, 63].
Altogether, topical treatments play a substantial role in the treatment of beyond-mild psoriasis. Novel topicals offer HCPs increasing flexibility to tailor treatments for patients with more severe psoriasis. The benefits of topicals may include reductions in dose-related adverse events as a result of lower systemic use.
Limitations
Our recommendations and consensus statements are based on data on short and long-term effectiveness, safety and current clinical use of Cal/BD foam for beyond-mild psoriasis patients. Some of the recommendations are possibly generalisable to other formulations or therapies, but they were not discussed at length. Now that this group of beyond-mild patients has been described, it would be useful to develop a pathway for them that addresses their clinical management more comprehensively.
Conclusion
Novel topical treatments may offer HCPs increased flexibility to tailor treatments for their patients with beyond-mild psoriasis. These recommendations are intended to help provide HCPs with guidance to support their use of the management of plaque psoriasis with topical treatment. Topical treatment with Cal/BD foam was qualified to be an appropriate alternative as monotherapy or as add-on treatment with non-biologic or biologic systemic therapy for beyond-mild psoriasis, and ultimately to optimise treatment outcomes for these patients.
References
Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–85.
Knuckles MLF, Levi E, Soung J. Defining and treating moderate plaque psoriasis: a dermatologist survey. J Dermatolog Treat. 2018;29:658–63.
Zill JM, Dirmaier J, Augustin M, Dwinger S, Christalle E, Härter M, et al. Psychosocial distress of patients with psoriasis: protocol for an assessment of care needs and the development of a supportive intervention. JMIR Res Protoc. 2018;7:e22.
Mrowietz U, de Jong EM, Kragballe K, Langley R, Nast A, Puig L, Reich K, Schmitt JWR. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2014;28:438–53.
Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643–59.
Samarasekera EJ, Sawyer L, Wonderling D, Tucker R, Smith CH. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br J Dermatol. 2013;168:954–67.
National Institute for Health and Care Excellence. NICE Pathways. Topical therapy for psoriasis. 2019.
Maul JT, Anzengruber F, Conrad C, Cozzio A, Häusermann P, Jalili A, et al. Topical treatment of psoriasis vulgaris: the Swiss treatment pathway. Dermatology. 2021;237:166–78.
Elmets CA, Korman NJ, Prater EF, Wong EB, Rupani RN, Kivelevitch D, et al. Joint AAD–NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432–70. https://doi.org/10.1016/j.jaad.2020.07.087.
National Institute for Health and Care Excellence. Psoriasis: assessment and management. Clinical guideline CG153. 2012.
Smith CH, Jabbar-Lopez ZK, Yiu ZZ, Bale T, Burden AD, Coates LC, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628–36.
Amin M, No DJ, Egeberg A, Wu JJ. Choosing first-line biologic treatment for moderate-to-severe psoriasis: what does the evidence say? Am J Clin Dermatol. 2018;19:1–13.
Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs. An Bras Dermatol. 2017;92:668–74.
Aschoff R, Bewley A, Martorell A. Real-world experience using topical therapy—calcipotriol and betamethasone dipropionate foam—in adults with beyond-mild psoriasis. Dermatol Ther. 2021;11:555–69. https://doi.org/10.1007/s13555-021-00501-3.
Chiricozzi A, Pitocco R, Saraceno R, Nistico S, Giunta A, Chimenti S. New topical treatments for psoriasis. Expert Opin Pharmacother. 2014;15:461–70.
Lebwohl M, Kircik L, Lacour J-P, Liljedahl M, Lynde C, Mørch MH, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52-weeks (PSO-LONG trial). J Am Acad Dermatol. 2021;84:1269–77.
Paul C, Leonardi C, Menter A, Reich K, Stein Gold L, Warren RB, et al. Calcipotriol plus betamethasone dipropionate aerosol foam in patients with moderate-to-severe psoriasis: sub-group analysis of the PSO-ABLE study. Am J Clin Dermatol. 2017;18:405–11.
Kircik L. Efficacy and safety of calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in combination with apremilast in patients with moderate plaque psoriasis. In: Presented at the 17th Annual South Beach Symposium, Miami Beach, February 7–10, 2019.
Bagel J, Gold L. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209–22.
Hasson F, Keeney SMH. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008–15.
Strober B, Ryan C, van de Kerkhof P, van der Walt J, Kimball AB, Barker J, et al. Re-categorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. 2020;82:117–22.
Mrowietz U, Kragballe K, Reich K, Spuls P, Griffiths CEM, Nast A, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1–10.
Papp K, Gulliver W, Lynde C, Poulin Y, Ashkenas J, Canadian Psoriasis Guidelines Committee. Canadian guidelines for the management of plaque psoriasis: overview. J Cutan Med Surg. 2011;15:210–9.
Leo Laboratories Limited. Enstilar 50 micrograms/g + 0.5 mg/g cutaneous foam. Summary of Product Characteristics. 2019.
Amatore F, Villani A, Tauber M. French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults. J Eur Acad Dermatol Venereol. 2019;33:464–83.
Paul C, Stein Gold L, Cambazard F, Kalb RE, Lowson D, Bang B, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol. 2017;31:119–26.
Frieder J, Kivelevitch D, Menter A. Calcipotriene betamethasone dipropionate aerosol foam in the treatment of plaque psoriasis: a review of the literature. Ther Deliv. 2017;8:737–46.
Menter A, Gold LS, Koo J, Villumsen J, Rosén M, Lebwohl M. Fixed-combination calcipotriene plus betamethasone dipropionate aerosol foam is well tolerated in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. Skinmed. 2017;15:119–24.
Pinter A, Thormann H, Angeletti F, Jalili A. Calcipotriol/betamethasone dipropionate aerosol foam for the treatment of psoriasis vulgaris: case series and review of the literature. Clin Cosmet Investig Dermatol. 2018;11:451–9.
Martin G, Young M, Aldredge L. Recommendations for initiating systemic therapy in patients with psoriasis. J Clin Aesthet Dermatol. 2019;12:13–26.
Buckinghamshire Healthcare NHS Trust. 738FM.8 Biologics for the treatment of psoriasis (adults). 2019. http://www.bucksformulary.nhs.uk/docs/Guideline_738FM.pdf.
Bae J-M. Shared decision making: relevant concepts and facilitating strategies. Epidemiol Health. 2017;39:e2017048.
Alinia H, Moradi Tuchayi S, Smith JA, Richardson IM, Bahrami N, Jaros SC, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759–64.
Reich K, Reich J, Falk T, Blödorn-Schlicht N, Mrowietz U, Kiedrowski R, et al. Clinical response of psoriasis to subcutaneous methotrexate correlates with inhibition of cutaneous Th1- and Th17-inflammatory pathways. Br J Dermatol. 2019;181:859–62.
Warren R, Mrowietz U, Kiedrowski R, Niesmann J, Wilsmann-Theis D, Ghoreschi K, et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:528–37.
Laws PM, Young HS. Update of the management of chronic psoriasis: new approaches and emerging treatment options. Clin Cosmet Investig Dermatol. 2010;3:25–37.
Kusumaningrum N, Wicaksono D, Winarni DR, Wirohadidjojo Y, Radiono S. Slow responder against methotrexate 50 mg intramuscular in severe psoriatic patients: a case series. Dermatol Rep. 2019;11:8080.
Maul J-T, Djamei V, Kolios AGA, Meier B, Czernielewski J, Jungo P, et al. Efficacy and survival of systemic psoriasis treatments: an analysis of the Swiss registry SDNTT. Dermatology. 2016;232:640–7.
Lebwohl MG, Bachelez H, Barker J, Girolomoni G, Kavanaugh A, Langley RG, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871–81.
Gordon K, Armstrong A, Wu J. Treating to target—a realistic goal in psoriasis? Semin Cutan Med Surg. 2018;37:S44–7.
Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70.
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6.
Koo K, Jeon C, Bhutani T. Beyond monotherapy: a systematic review on creative strategies in topical therapy of psoriasis. J Dermatolog Treat. 2017;28:702–8.
Jensen JD, Delcambre MR, Nguyen G, Sami N. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379–85.
Gelfand JM, Wan J, Callis Duffin K, Krueger GG, Kalb RE, Weisman JD, et al. Comparative effectiveness of commonly used systemic treatments or phototherapy for moderate to severe plaque psoriasis in the clinical practice setting. Arch Dermatol. 2012;148:487–94.
Lebwohl M, Kircik L, Callis Duffin K, Pariser D, Hooper M, Wenkert D, Thompson E, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385–92.
Yao C, Lebwohl M. Onset of action of antipsoriatic drugs for moderate-to-severe plaque psoriasis: an update. J Drugs Dermatol. 2019;18:229–33.
Thaçi D, Ortonne J-P, Chimenti S, Ghislain P-D, Arenberger P, Kragballe K, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402–11.
Iversen L, Lange M, Bissonette R, Carvalho A, van de Kerkhof P, Kirby B, et al. Topical treatment of psoriasis: questionnaire results on topical therapy accessibility and influence of body surface area on usage. J Eur Acad Dermatol Venereol. 2017;31:1188–95.
Richardson SK, Gelfand JM. Update on the natural history and systemic treatment of psoriasis. Adv Dermatol. 2008;24:171–96.
Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–50.
Lin P-T, Wang S-H, Chi C-C. Drug survival of biologics in treating psoriasis: a meta-analysis of real-world evidence. Sci Rep. 2018;8:16068.
Egeberg A, Ottosen M, Gniadecki R, Broesby-Olsen S, Dam T, Bryld L, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178:509–19.
Karakawa M, Komine M, Kishimoto M, Maki N, Matsumoto A, Sugai J, et al. Effects of maxacalcitol ointment on skin lesions in patients with psoriasis receiving treatment with adalimumab. J Dermatol. 2016;43:1354–7.
Belinchón I, Rivera R, Blanch C, Comellas M, Lizán L. Adherence, satisfaction and preferences for treatment in patients with psoriasis in the European Union: a systematic review of the literature. Patient Prefer Adherence. 2016;10:2357–67.
van der Schoot L, van den Reek J, Groenewoud J, Otero M, Njoo M, Ossenkoppele P, et al. Female patients are less satisfied with biological treatment for psoriasis and experience more side-effects than male patients: results from the prospective BioCAPTURE registry. J Eur Acad Dermatol Venereol. 2019;33:1913–20.
Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:845–50.
Volc S, Ghoreschi K. Pathophysiological basis of systemic treatments in psoriasis. J Dtsch Dermatol Ges. 2016;14:557–72.
Gisondi P, Del Giglio M, Girolomoni G. Treatment approaches to moderate to severe psoriasis. Int J Mol Sci. 2017;18:2427.
Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628–31.
Feldman SR, Goffe B, Rice G, Mitchell M, Kaur M, Robertson D, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Heal Drug Benefits. 2016;9:504–13.
Wu J, Armstrong A, Gordon K. Practical strategies for optimizing management of psoriasis. Semin Cutan Med Surg. 2018;37:S52–5.
Fowler JF, Del Rosso JQ, Pakunlu RI, Sidgiddi S. Treatment satisfaction, product perception, and quality of life in plaque psoriasis patients using betamethasone dipropionate spray 0.05. J Clin Aesthet Dermatol. 2017;10:13–8.
Acknowledgements
Funding
This document was drafted with the input of the authors who attended a consensus development workshop convened by LEO Pharma A/S, Denmark, which was held in Copenhagen, Denmark on 4 September 2019. Advisors received no honoraria from LEO Pharma A/S, Denmark for activities related to this publication; however, reasonable travel and accommodation expenses for the original meeting were reimbursed. The journal’s Rapid Service Fee was also funded by LEO Pharma A/S.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
RA, AB, AD, CDS, ML, MLV, AM, MP and MS generated and reviewed the statements for the Delphi process, and were involved in reaching consensus statements. RA, AB, AD, CDS, ML, MLV, AM, MP and MS provided critical input to the manuscript, reviewed drafts and approved the final version for publication.
Medical Writing Assistance
Medical writing support was provided by Cello Health Communications (Andrea Hamlin, PhD, Rudy Sarmah, BSc and Anthony Zucker, PhD) and was funded by LEO Pharma A/S Ballerup, Denmark.
Disclosures
Roland Aschoff reports personal fees for participation in advisory boards and lectures from Biofrontera and LEO Pharma, and personal fees for lecture participation from AbbVie, Galderma, Mylan and Sanofi. Anthony Bewley reports a research grant from the European Academy of Dermatology Venereology for Practical Psychodermatology; ad hoc consultancy and travel grants from Almirall, Janssen and LEO Pharma; and consultancy fees from AbbVie, Almirall, Celgene, Galderma, Janssen, LEO Pharma, Eli Lilly, Novartis, Sanofi, UCB Pharma. He is also Treasurer and a member of guidelines committees for the British Association of Dermatology, Secretary of the European Society for Dermatology and Psychiatry, Chair of the All-Party Parliamentary Group on Skin, and acts as an Advisor for the Psoriasis Association, Changing Faces, Ichthyosis Support Group and the National Eczema Society. Annunziata Dattola has been a consultant and/or speaker for UCB Pharma, Eli Lilly, LEO Pharma, Medac Pharma, Johnson & Johnson, and Pierre Fabre. Clara De Simone has received personal fees for acting as board member and/or speaker from AbbVie, Almirall, Celgene, LEO Pharma, Eli Lilly, Janssen, Novartis, Sanofi and UCB Pharma. Mourad Lahfa reports personal fees, as investigator, consultant, and speaker, from Novartis, AbbVie, Pierre Fabre Dermatology, Celgene, Amgen, Janssen-Cilag, Pfizer, LEO Pharma, Galderma, Eli Lilly, Sanofi, and UCB Pharma. Mar Llamas-Velasco reports personal fees for participation as an advisory board member, consultant and/or speaker, and grants/research support for participation in clinical trials with AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Eli Lilly, Novartis and UCB Pharma, not related to the submitted work. Antonio Martorell has received personal fees and non-financial support from AbbVie, Janssen, UCB Pharma, and Eli Lilly, and personal fees from Novartis, LEO Pharma and MSD. Mira Pavlovic has nothing to disclose. Michael Sticherling was an advisor and/or received honoraria for speaking and/or received grants and/or participated in clinical trials for AbbVie, Actelion, Amgen, Celgene, Eli Lilly, Galderma, GSK, Janssen-Cilag, LEO Pharma, MSD, Mundipharma, Novartis, Pfizer, Regeneron, Sanofi and UCB Pharma.
Compliance with Ethics Guidelines
This paper is formed of the opinions of the authors themselves and contains no research or study elements that would require ethics committee approval.
Data Availability
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Aschoff, R., Bewley, A., Dattola, A. et al. Beyond-Mild Psoriasis: A Consensus Statement on Calcipotriol and Betamethasone Dipropionate Foam for the Topical Treatment of Adult Patients. Dermatol Ther (Heidelb) 11, 1791–1804 (2021). https://doi.org/10.1007/s13555-021-00600-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-021-00600-1