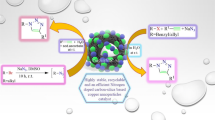
Abstract
A facile and highly efficient protocol for 1,3-dipolar cycloaddition of in situ generated nitrile oxides with terminal alkynes catalyzed by copper-doped silica cuprous sulfate (CDSCS) as a new and convenient heterogeneous nano catalyst is described. In this protocol, ‘click’ cycloaddition of various structurally diverse alkynes and imidoyl chlorides in the presence of CDSCS and NaHCO3 in a solution of i-PrOH/H2O (1:1, V/V) furnishes the corresponding 3,5-disubstituted isoxazoles in good to excellent yields at room temperature. CDSCS was approved as a chemically and thermally stable nano catalyst that can be recovered and reused for many consecutive trials without considerable decline in its reactivity.

Similar content being viewed by others
References
A. Kleeman, J. Engel, B. Kutscher, D. Reichert, Pharmaceutical substances, 3rd edn. (Thieme, Stuttgart, 1999)
J. Sperry, D. Wright, Curr. Opin. Drug Discov. Dev. 8, 723 (2005)
E.M. Pinho, M.V.D. Teresa, Curr. Org. Chem. 9, 925 (2005)
P.G. Baraldi, A. Barco, S. Benetti, G.P. Pollini, D. Simoni, Synthesis 857 (1987)
P. Conti, C. Dallanoce, M.D. Amici, C.D. Micheli, K.-N. Klotz, Bioorg. Med. Chem. 6, 401 (1998)
A. Mishra, S.K. Jain, J.G. Asthana, Orient. J. Chem. 14, 151 (1998)
D.-H. Ko, M.F. Maponya, M.A. Khalil, E.T. Oriaku, Z. You, J. Lee, Med. Chem. Res. 8, 313 (1998)
J.J. Talley, D.L. Brown, J.S. Carter, M.J. Graneto, C.M. Koboldt, J.L. Masferrer, W.E. Perkins, R.S. Rogers, A.F. Shaffer, Y.Y. Zhang, B.S. Zweifel, K. Seibert, J. Med. Chem. 43, 775 (2000)
Y.Y. Kang, K.J. Shin, K.H. Yoo, K.J. Seo, C.Y. Hong, C.-S. Lee, S.Y. Park, D.J. Kim, S.W. Park, Bioorg. Med. Chem. Lett. 10, 95–99 (2000)
Y.-S. Lee, B.H. Kim, Bioorg. Med. Chem. Lett. 12, 1395 (2002)
S. Srivastava, L.K. Bajpai, S. Batra, A.P. Bhaduri, J.P. Maikhuri, G. Gupta, J.D. Dhar, Bioorg. Med. Chem. 7, 2607 (1999)
K.D. Shin, M.Y. Lee, D.S. Shin, S. Lee, K.H. Son, S. Koh, Y.K. Paik, B.M. Kwon, D.C. Han, J. Biol. Chem. 280, 41439 (2005)
B.L. Deng, M.D. Cullen, Z. Zhou, T.L. Hartman, R.W. Buckheit Jr, C. Pannecouque, E. Declescq, P.E. Fanwick, M. Cushman, Bioorg. Med. Chem. 14, 2366 (2006)
X. Han, C. Li, K.C. Rider, A. Blumenfeld, B. Twamley, N.R. Natale, Tetrahedron Lett. 43, 7673 (2002)
K.E. Pallet, S.M. Cramp, J.P. Little, P. Veerasekaran, A.J. Crudace, A.E. Slater, Pest Manag. Sci. 57, 133 (2001)
U.S. Sørensen, E. Falch, P. Krogsgaard-Larsen, J. Org. Chem. 65, 1003 (2000)
D. Raffa, G. Daidone, B. Maggio, D. Schillaci, F. Plescia, L. Torta, Farmaco 54, 90 (1999)
A.P. Kozikowski, P.D. Stein, J. Am. Chem. Soc. 104, 4023 (1982)
D.P. Curran, J. Am. Chem. Soc. 105, 5826 (1983)
B.H. Kim, Y.J. Chung, E.J. Ryu, Tetrahedron Lett. 34, 8465 (1993)
J.W. Bode, E.M. Carreira, Org. Lett. 3, 1587 (2001)
A.P. Kozikowski, Y.Y. Chen, J. Org. Chem. 46, 5248 (1981)
I. Muller, V. Jager, Tetrahedron Lett. 23, 4777 (1982)
V. Jager, H. Grund, Angew. Chem. Int. Ed. Engl. 15, 50 (1976)
S.Y. Lee, B.S. Lee, C.-W. Lee, D.Y. Oh, J. Org. Chem. 65, 256 (2000)
G.W. Moersch, E.L. Wittle, W.A. Neuklis, J. Org. Chem. 32, 1387 (1967)
A. Yashiro, Y. Nishida, K. Kobayashi, M. Ohno, Synlett 361 (2000)
J. Barbera, C. Cativiela, J.L. Serrano, M.Z. Zurbano, Liq. Cryst. 11, 887 (1992)
D.H. Brown, P. Styring, Liq. Cryst. 30, 23 (2003)
A.R. Katritzky, M. Wang, S. Zhang, M.V. Voronkov, J. Org. Chem. 66, 6787 (2001)
T. Bandiera, P. Grunanger, A.F. Marinone, J. Heterocycl. Chem. 29, 1423 (1992)
S.S. Chauhan, Y.C. Joshi, Rasayan J. Chem. 1, 475 (2008)
S. Tang, J. He, Y. Sun, L. He, X. She, Org. Lett. 11, 3982 (2009)
M.S.M. Ahmed, K. Kobayashi, A. Mori, Org. Lett. 7, 4487 (2005)
T. Ghosh, C. Bandyopadhyay, Tetrahedron Lett. 45, 6169 (2004)
P. Cuadrado, A.M. González-Nogal, R. Valero, Tetrahedron 58, 4975 (2002)
C. Praveen, A. Kalyanasundaram, P.T. Perumal, Synlett 777 (2010)
M. Ueda, Y. Ikeda, A. Sato, Y. Ito, M. Kakiuchi, H. Shono, T. Miyoshi, T. Naito, O. Miyata, Tetrahedron 67, 4612 (2011)
J.P. Waldo, R.C. Larock, Org. Lett. 7, 5203 (2005)
J.P. Waldo, R.C. Larock, J. Org. Chem. 72, 9643 (2007)
M. Ueda, A. Sato, Y. Ikeda, T. Miyoshi, T. Naito, O. Miyata, Org. Lett. 12, 2594 (2010)
G.D. Vilela, R.R. da Rosa, P.H. Schneider, I.H. Bechtold, J. Eccher, A.A. Merlo, Tetrahedron Lett. 52, 6569 (2011)
R. Li, W.T. Wu, G.L. Wu, Y. Fan, L.M. Wu, Chin. Chem. Lett. 18, 788 (2007)
P. Grünanger, P. Vita-Finzi, in The chemistry of heterocyclic compounds: isoxazoles, Part I, vol. 49, ed. by E.C. Taylor, A. Weissberger (Wiley, New York, 1991), pp. 1–416
K.B.G. Torssel, Nitrile oxides, nitrones, and nitronates in organic synthesis (VCH, New York, 1988)
R. Huisgen, in 1,3-Dipolar cycloaddition chemistry, vol. 1, ed. by A. Padwa (Wiley, New York, 1984), pp. 1–176
V.P. Sandanayaka, Y. Youjun, Org. Lett. 2, 3087 (2000)
P.D. Croce, C. La Rosa, G. Zecchi, J. Chem. Soc. Perkin Trans. 1, 2621 (1985)
F. Himo, T. Lovell, R. Hilgraf, V.V. Rostovtsev, L. Noodleman, K.B. Sharpless, V.V. Fokin, J. Am. Chem. Soc. 127, 210 (2005)
T.V. Hansen, P. Wu, V.V. Fokin, J. Org. Chem. 70, 7761 (2005)
A.A. Vieira, E.R. Bryk, G. Conte, A.J. Bortoluzzi, H. Gallardo, Tetrahedron Lett. 50, 905 (2009)
M.N. Soltani Rad, S. Behrouz, M.M. Doroodmand, A. Movahediyan, Tetrahedron 68, 7812 (2012)
M. Tanaka, T. Haino, K. Ideta, K. Kubo, A. Mori, Y. Fukazawa, Tetrahedron 63, 652 (2007)
Acknowledgments
We are grateful to Shiraz University of Technology Research Council for partial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soltani Rad, M.N., Behrouz, S. & Faghihi, M.A. Copper-doped silica cuprous sulfate (CDSCS) as a highly efficient heterogeneous nano catalyst for synthesis of 3,5-disubstituted isoxazoles. J IRAN CHEM SOC 11, 361–367 (2014). https://doi.org/10.1007/s13738-013-0307-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-013-0307-4