Abstract
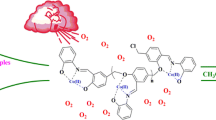
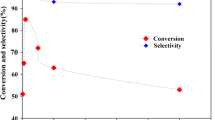
The present work introduced the new strategy for direct preparation of Schiff base as well as oxime compounds through oxidation of primary benzylic or allylic alcohols in the presence of amines by complexation of Mn(III) to a polymeric Schiff base ligand based on polysalicylaldehyde (PSA-Schiff base-Mn(III) complex). As a new environmentally benign protocol, manganese heterogeneous polymeric catalytic system demonstrated promising oxidation of alcohols in ethanol using molecular oxygen. PSA was synthesized through polycondensation reaction of 2-hydroxy-5-chloromethyl-benzaldehyde and then treated with 2-aminophenol to form polymeric ligand. Average molecular weight of PSA was studied by an analytical method as well as GPC analysis. Formation of the catalyst was characterized step by step by FTIR, UV–Vis, 1H NMR, TGA, CHN and EDX analyses. Loading amounts of metal ions as well as leaching amount of the catalysis were studied by ICP-OES instrument. The catalyst shows up to high yields for oxidation of primary and secondary primary benzylic or allylic alcohols to carbonyl compounds, especially direct imine formation in a mild, inexpensive and efficient method which can be successfully recovered from the reaction mixture and reused for several times without any remarkable reactivity loss. Effect of solvent, temperature, catalyst amount and oxygen donors along with some blank experiments to elucidation of catalyst activity was evaluated in this work. Also chemoselectivity behavior of the catalyst was investigated with some combinations.










Similar content being viewed by others
References
M. Ghassemzadeh, B. Rezaeirad, S. Bahemmat, B. Neumüller, J. Iran. Chem. Soc. 9, 285 (2012)
K. Poonia, S. Siddiqui, M. Arshad, D. Kumar, Spectrochim. Acta A Mol. Biomol. Spectrosc. 155, 146 (2016)
A. D. Khalaji, H. Mighani, M. Kazemnejadi, K. Gotoh, H. Ishida, K. Fejfarova M. Dusek, Arab. J. Chem. (2013), in press
J.R. Anacona, N. Noriega, J. Camus, Acta A Mol. Biomol. Spectrosc. 137, 16 (2015)
H. Keypour, M.H. Zebarjadian, M. Rezaeivala, M. Shamsipur, S.J. Sabounchei, J. Iran. Chem. Soc. 10, 1137 (2013)
H. Keypour, M. Liyaghati-Delshad, M. Rezaeivala, M. Bayat, J. Iran. Chem. Soc. 12, 621 (2015)
C.M. da Silva, D.L. da Silva, L.V. Modolo, R.B. Alves, M.A. de Resende, C.V. Martins, Â. de Fátima, J. Adv. Res. 2, 1 (2011)
K.S. Kumar, S. Ganguly, R. Veerasamy, E. De Clercq, Eur. J. Med. Chem. 45, 5474 (2010)
S.N. Pandeya, A.S. Raja, J.P. Stables, J. Pharm. Pharm. Sci. 5, 266 (2002)
S. Ren, R. Wang, K. Komatsu, P. Bonaz-Krause, Y. Zyrianov, C.E. McKenna, C. Csipke, Z.A. Tokes, E.J. Lien, J. Med. Chem. 45, 410 (2002)
Z. Asadi, M. Asadi, F.D. Firuzabadi, R. Yousefi, M. Jamshidi, J. Iran. Chem. Soc. 11, 423 (2014)
S.P. Chatterjee, B. Sur, S.R. Chaudhary, Oncology 47, 433 (1990)
S. Adsule, V. Barve, D. Chen, F. Ahmed, Q.P. Dou, S. Padhye, F.H. Sarkar, J. Med. Chem. 49, 7242 (2006)
H. Polasa, Indian J. Pharm. Sci. 47, 202 (1985)
S. Ershad, L. Sagathforoush, G. Karim-Nezhad, S. Kangari, Int. J. Electrochem. Sci. 4, 846 (2009)
J. Gradinaru, A. Forni, V. Druta, F. Tessore, S. Zecchin, S. Quici, N. Garbalau, Inorg. Chem. 46, 884 (2007)
E. Lindbäck, H. Norouzi-Arasi, E. Sheibani, D. Ma, S. Dawaigher, K. Wärnmark, ChemistrySelect 1, 1789 (2016)
M. Salavati-Niasari, F. Davar, M. Bazarganipour, Dalton Trans. 39, 7330 (2010)
K. Tiwari, M. Mishra, V.P. Singh, RSC Adv. 4, 27556 (2014)
N. Nishat, S. Hasnain, T. Ahmad, A. Parveen, J. Therm. Anal. Calorim. 105, 969 (2011)
L. Moradi, M. Bina, T. Partovi, Curr. Chem. Lett. 3, 147 (2014)
M. Salavati-Niasari, E. Esmaeili, H. Seyghalkar, M. Bazarganipour, Inorg. Chim. Acta 375, 11 (2011)
M. Salavati-Niasari, M. Bazarganipour, Appl. Surf. Sci. 255, 2963 (2008)
A. Bilici, F. Doğan, I. Kaya, Ind. Eng. Chem. Res. 53, 104 (2013)
A. Bi̇li̇ci̇, İ. Kaya, F. Doğan, J. Polym. Sci., Part A: Polym. Chem. 47, 2977 (2009)
S. Waśkiewicz, K. Zenkner, E. Langer, M. Lenartowicz, I. Gajlewicz, Prog. Org. Coat. 76, 1040 (2013)
E. Zhang, H. Tian, S. Xu, X. Yu, Q. Xu, Org. Lett. 15, 2704 (2013)
J.T. Reeves, M.D. Visco, M.A. Marsini, N. Grinberg, C.A. Busacca, A.E. Mattson, C.H. Senanayake, Org. Lett. 17, 2442 (2015)
Q. Kang, Y. Zhang, Green Chem. 14, 1016 (2012)
L. Jiang, L. Jin, H. Tian, X. Yuan, X. Yu, Q. Xu, Chem. Commun. 47, 10833 (2011)
H. Tian, X. Yu, Q. Li, J. Wang, Q. Xu, Adv. Synth. Catal. 354, 2671 (2012)
M.S. Kwon, S. Kim, S. Park, W. Bosco, R.K. Chidrala, J. Park, J. Org. Chem. 74, 2877 (2009)
S. Sithambaram, R. Kumar, Y.C. Son, S.L. Suib, J. Catal. 253, 269 (2008)
H.K. Kwong, P.K. Lo, K.C. Lau, T.C. Lau, Chem. Commun. 47, 4273 (2011)
H. Tsunoyama, H. Sakurai, Y. Negishi, T. Tsukuda, J. Am. Chem. Soc. 127, 9374 (2005)
D.K.T. Yadav, B.M. Bhanage, RSC Adv. 5, 12387 (2015)
A.R. Hajipour, S.E. Mallakpour, I. Mohammadpoor-Baltork, S. Khoee, Chem. Lett. 2, 120 (2000)
E.P. Talsi, K.P. Bryliakov, Coord. Chem. Rev. 256, 1418 (2012)
K. Schröder, K. Junge, B. Bitterlich, M. Beller, Top. Organomet. Chem. 33, 83 (2011)
A. Onopchenko, J. Schulz, J. Org. Chem. 40, 3338 (1975)
C.C. Cosner, P.J. Cabrera, K.M. Byrd, A.M.A. Thomas, P. Helquist, Org. Lett. 13, 2071 (2011)
H.Y. Sun, Q. Hua, F.F. Guo, Z.Y. Wang, W.X. Huang, Adv. Synth. Catal. 354, 569 (2012)
T.K.M. Shing, Y.Y. Yeung, P.L. Su, Org. Lett. 8, 3149 (2006)
P.G. Cozzi, Chem. Soc. Rev. 33, 410 (2004)
K. Srinivasan, P. Michaud, J.K. Kochi, J. Am. Chem. Soc. 108, 2309 (1986)
T.C. Mac Leod, M.V. Kirillova, A.J. Pombeiro, M.A. Schiavon, M.D. Assis, Appl. Catal. A Gen. 372, 191 (2010)
N.C. Gianneschi, P.A. Bertin, S.T. Nguyen, C.A. Mirkin, L.N. Zakharov, A.L. Rheingold, J. Am. Chem. Soc. 125, 10508 (2003)
S. Sithambaram, R. Kumar, Y.C. Son, S.L.J. Suib, Catal. 253, 269 (2008)
B. Chen, J. Li, W. Dai, L. Wang, S. Gao, Green Chem. 16, 3328 (2014)
S. Biswas, B. Dutta, K. Mullick, C.H. Kuo, A.S. Poyraz, S.L. Suib, ACS Catal. 5, 4394 (2015)
M.A. Nasseri, A. Mohammadinezhad, M. Salimi, J. Iran. Chem. Soc. 12, 81 (2015)
M.T. Räisänen, A. Al-Hunaiti, E. Atosuo, M. Kemell, M. Leskelä, T. Repo, Catal. Sci. Technol. 4, 2564 (2014)
B. Chen, L. Wang, S. Gao, ACS Catal. 5, 5851 (2015)
Food Cosmet, Toxicol. 17, 903 (1979)
D. Janeš, S. Kreft, Food Chem. 109, 293 (2008)
R. Tang, F.X. Webster, D. Müller-Schwarze, J. Chem. Ecol. 19, 1491 (1993)
A. Dehno Khalaji, M. Kazemnejadi, H. Mighani, D. Das, J. App. Chem. 7, 77 (2013)
Q. Wang, C. Wilson, A.J. Blake, S.R. Collinson, P.A. Tasker, M. Schröder, Tetrahedron Lett. 47, 8983 (2006)
M. Pramanik, S.K. Mendon, J.W. Rawlins, Polym. Test. 31, 716 (2012)
V.P. Boiko, V.K. Grischenko, Rev. Acta Polym. 36, 459 (1985)
J.F. Zemaitis Jr., D.M. Clark, M. Rafal, N.C. Scrivner, Handbook of Aqueous Electrolyte Thermodynamics: Theory & Application (Wiley, New York, 1986), pp. 577–579
İ. Şakiyan, N. Gündüz, T. Gündüz, Synth. React. Inorg. Met. Org. Chem. 31, 1175 (2001)
İ. Şakıyan, Transit. Met. Chem. 32, 131 (2007)
S.M.D.M. Romanowski, S.P. Machado, G.R. Friedermann, A.S. Mangrich, M.D.F. Hermann, H.O. Lima, S. Nakagaki, J. Braz. Chem. Soc. 21, 842 (2010)
D.R. Learman, B.M. Voelker, A.S. Madden, C.M. Hansel, Front. Microbial. 4, 262 (2013)
J.E. Jee, O. Pestovsky, A. Bakac, Dalton Trans. 39, 11636 (2010)
B. Bahramian, V. Mirkhani, M. Moghadam, A.H. Amin, Appl. Catal. A Gen. 315, 52 (2006)
M.M. Najafpour, M.Z. Ghobadi, B. Sarvi, B. Haghighi, Dalton Trans. 44, 15271 (2015)
H.R. Godini, A. Gili, O. Görke, S. Arndt, U. Simon, A. Thomas, R. Schomäcker, G. Wozny, Catal. Today 236, 12 (2014)
A.A.A. Aziz, A.N.M. Salem, M.A. Sayed, M.M. Aboaly, J. Mol. Struct. 1010, 130 (2012)
V.D. Chaube, S. Shylesh, A.P. Singh, J. Mol. Catal. A: Chem. 241, 79 (2005)
S. Shylesh, A.P. Singh, J. Catal. 228, 333 (2004)
S. Shylesh, S. Sharma, S.P. Mirajkar, A.P. Singh, J. Mol. Catal. A: Chem. 212, 219 (2004)
T. Joseph, D.P. Sawant, C.S. Gopinath, S.B. Halligudi, J. Mol. Catal. A: Chem. 184, 289 (2002)
P. Karandikar, M. Agashe, K. Vijaymohanam, A.J. Chandwadkar, Appl. Catal. A Gen. 257, 133 (2004)
A. Tashiro, A. Mitsuishi, R. Irie, T. Katsuki, Synlett 2003, 1868 (2003)
H. Tanak, M. Macit, M. Yavuz, Ş. Işık, Acta Crystallogr. Sect. E: Struct. Rep. Online 65, o3056 (2009)
D. Şahin, S. Koçoğlu, Ö. Şener, C. Şenol, H. Dal, T. Hökelek, Z. Hayvalı, J. Mol. Struct. 1102, 302 (2015)
K.W. Mukonyi, I.O. Ndiege, Bull. Chem. Soc. Ethiop. 15, 137 (2001)
A.J. Bosco, S. Lawrence, C. Christopher, S. Radhakrishnan, J. Rosario, A. Arul, S. Raja, D. Vasudevan, J. Phys. Org. Chem. 28, 591 (2015)
A.R. Katritzky, M. Szajda, S. Bayyuk, Synthesis 1986, 804 (1986)
T.M. Fasina, O.O. Ogundele, I. Ayeni, J. Chem. Pharm. Res. 6, 816 (2014)
E.C. Niederhoffer, J.H. Timmons, A.E. Martell, Chem. Rev. 84, 137 (1984)
M.B. Fugu, N.P. Ndahi, B.B. Paul, A.N. Mustapha, J. Chem. Pharm. Res. 5, 22 (2013)
K. Guo, Y. Chen, Anal. Methods 2, 1156 (2010)
İ. Kaya, S. Çulhaoğlu, Chin. J. Polym. Sci. 26, 131 (2008)
L. Saikia, J.M. Baruah, A.J. Thakur, Org. Med. Chem. Lett. 1, 12 (2011)
L. Dinparast, H. Valizadeh, Iran. J. Catal. 5, 73 (2015)
J. Yu, M. Lu, Synlett 25, 1873 (2014)
X. Liang, Z. Mi, Y. Wang, L. Wang, X. Zhang, React. Kinet. Catal. Lett. 82, 333 (2004)
R.H. Crabtree, Chem. Rev. 112, 1536 (2012)
M. Esmaeilpour, J. Javidi, M. Divar, J. Magn. Magn. Mater. 423, 232 (2017)
M. Esmaeilpour, J. Javidi, S. Zahmatkesh, Appl. Organomet. Chem. 30, 897 (2016)
B. Gnanaprakasam, J. Zhang, D. Milstein, Angew. Chem. Int. Ed. Engl. 122, 1510 (2010)
S. Kegnæs, J. Mielby, U.V. Mentzel, C.H. Christensen, A. Riisager, Green Chem. 12, 1437 (2010)
L. Blackburn, R.J. Taylor, Org. Lett. 3, 1637 (2001)
Acknowledgement
The authors are grateful to Golestan University research council for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kazemnejadi, M., Shakeri, A., Mohammadi, M. et al. Direct preparation of oximes and Schiff bases by oxidation of primary benzylic or allylic alcohols in the presence of primary amines using Mn(III) complex of polysalicylaldehyde as an efficient and selective heterogeneous catalyst by molecular oxygen. J IRAN CHEM SOC 14, 1917–1933 (2017). https://doi.org/10.1007/s13738-017-1131-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1131-z