Abstract
Many urban water bodies in Chennai, India receive untreated sewage that pollutes their waters. An example is the Sembakkam lake, in which water reaches the Pallikaranai marshland, a proposed Ramsar site. In 2019, the city experienced the worst water crisis in 30 years, and many lakes were extremely dry, favoring peaks of heavy metals. Therefore, this study focuses on analysing heavy metal pollution and evaluating its potential effects on biota. In situ parameters were measured and water, sediment, and water hyacinth samples were collected during four campaigns. Al, As, Cr, Cu, Fe, Mn, Ni, Pb, and Zn were measured in all samples. Digestions for total metal content were performed in solid samples and acetic acid extractions only in sediments. The average pH (7.89–8.46) was neutral-alkaline and electrical conductivities (1559–2864 µS/cm) were high. In water, Pb (average: 2.59 µg/l) posed the highest toxicity risk according to the United Nations Economic Commission for Europe, followed by Cu and Cr. In sediment, Cu and Cr reached severe enrichment with respect to continental crust (averages: 19.46 and 13.65) followed by Ni and Zn with moderately severe enrichment. Ni produced the highest toxicity risk (average: 76.18 mg/kg), above the effects range-median, followed by Cr and Cu, between the effects range-low and effects range-median. The highest bioaccumulation factors in the water hyacinth were in the roots. Translocation factors showed similar concentrations in stems and leaves. Proper management of sewage is necessary to diminish the potential deleterious effects of metals on aquatic life and by extension, human health.
Similar content being viewed by others
Introduction
The United Nations defined the sustainable development goal number 6 “ensuring availability and sustainable management of water and sanitation for all” as one of the 17 Sustainable Development Goals to be achieved by 2030 (United Nations 2015). It includes the target 6.6 “protect and restore water-related ecosystems, including mountains, forests, wetlands, rivers, aquifers and lakes” (United Nations 2015). However, aquatic ecosystems are threatened by a variety of pressures such as water abstraction, pollution, invasive species, pathogens, geomorphological alterations, encroachment, and climate change, among others due to population growth and industrialization (Pistocchi et al. 2017; Udias et al. 2020).
This is the situation of many urban water bodies in developing countries, including in megacities, where larger numbers of humans are exposed to pollutants. A common situation in these countries is that untreated sewage is discharged into urban water bodies because of non-existing or non-properly-enforced regulations (Fraga et al. 2020; Kumar et al. 2022; Das et al. 2022). In this context, heavy metal concentrations can increase in water, sediments and living beings due to bioconcentration and biomagnification and become a risk for human communities nearby. In fact, some studies have alerted about this situation in many developing countries, like Egypt (Abu El-Magd et al. 2021), Malaysia (Prasanna et al. 2012) and China (Jiang et al. 2018). However, information on possible heavy metal related risks for humans in the urban context is still scarce.
India has experienced a rapid increase in population, a significant economic growth and an intense industrialization. After a protectionist period, the economy of India was liberalized in the 1990’s and it became the world's fastest growing major economy between 2014 and 2018 (International Monetary Fund 2019). This situation together with frequent discharges of untreated wastewater due to a lack of sewage treatment and control over the industrial sector caused many aquatic ecosystems to be polluted and their aquatic life to be reduced (Kelkar et al. 2011; Chaturvedi 2012; Hossain et al. 2013; Sharma et al. 2018). As an example, the sewage generation in the country was estimated to be around 62000 million liters per day (MLD) in 2015 while the installed sewage treatment capacity was only 23277 MLD (Sharawat et al. 2019). Therefore, discharge of untreated sewage into water bodies may be responsible for polluting three quarters of surface water resources (Kumar and Tortajada 2020).
Chennai, the capital of the state of Tamil Nadu, has also experienced intense population and industrial growth. Untreated domestic and industrial wastewater discharges (Arappor Iyakkam 2017), garbage dumping, and others are threatening the biodiversity of the city's large number of water bodies (Lan et al. 2014). They include the Pallikaranai marshland, a proposed Ramsar site that was drained to expand the urban area of the city and, consequently, reduced from around 50 km2 in 1980 to only 6 km2 today (Vencatesan 2007; Steinbruch and Hörmann 2015; Sree Sharmila and Swathika 2016). In addition, many artificial lakes, locally called tanks, are located within Chennai. The tanks are traditional retention storages that accumulated water during the monsoon season that was used for irrigation during the dry season (Devi et al. 2020). The tanks were usually constructed by damming intermittent streams using crescent-shaped earthen bunds in a cascade down the axes of shallow inland valleys (Massuel et al. 2014; Devi et al. 2020). However, water tanks became ornamental rather than functional when water provision shifted to groundwater, resulting in their abandonment and degradation (Palanisami et al. 2008; Adelina 2015). It also affected the tanks in the Pallikaranai catchment, including the Sembakkam lake, which waters reach the Pallikaranai Marshland. In the Adyar catchment, siltation diminished water storage capacity of their tanks by 15% (Massuel et al. 2014).
Some studies have reported on the worrying levels of heavy metals (in this article, the term heavy metal includes metals like Al, As, Cr, Cu, Fe, Mn, Ni, Pb, and Zn, measured in this work, and metalloids, i.e. As, etc.) in the water bodies of Chennai. In Chemberambakkam lake, where water is collected for drinking water supply, Cd, Pb, Fe, Co, and Ni in water were higher than the World Health Organization thresholds for drinking water (Prabhu et al. 2015). Water in some lakes of south Chennai has high concentrations of Cu and Pb (Lakshmi et al. 2018). In the Ennore creek, abnormalities attributed to heavy metals were found on the Asian green mussel Perna viridis (Vasanthi et al. 2017) and high concentrations of Fe, Mn, Zn, Cu, Pb, and Cd were found in the flathead grey mullet fish Mugil cephalus (Arockia Vasanthi et al. 2013). Jayaprakash et al. (2010) studied the sediments of the Pallikaranai marshland and found they are more heavily contaminated with Cd, Hg, Cr, Cu, Ni, Pb, and Zn than in other regions on the southeast coast of India. In beach sediments of Chennai, Pb and Ni were over the lowest effect level (LEL) and effects range low (ERL) in several locations (Santhiya et al. 2011). The water hyacinth Pontederia crassipes (formerly Eichhornia crassipes) is a plant that can concentrate heavy metals in its tissues and it is even used in constructed wetlands (Newete et al. 2016). Therefore, it can be used as an indicator or heavy metal pollution in waters (Eid et al. 2020).
Heavy metals in water bodies can reach higher concentrations than usual during drought events. Chennai suffers from drought regularly and it is ranked as the fourth most vulnerable city to climate change in India (Kelkar et al. 2011). In the intense drought event of 2019, many tanks were extremely dry and showed the lowest water area of the period 2015–2020. The surface of the largest tanks shrank significantly and some small tanks lost all their water (Jayaraman 2019; Water crisis in Chennai 2019).
Hence, this study aims to analyze the heavy metal pollution in water, sediments, and water hyacinths of the Sembakkam lake and to evaluate their potential toxicity risks on the biota during 2019, including an extreme drought event. Therefore, this study is a valuable example for other urban lakes in Chennai and Asia that suffer from similar pollution and climatic problems and contributes to a better understanding of the current water challenges in the region, and helps stakeholders to implement management strategies to comply with the Sustainable Development Goals.
Materials and methods
Study area
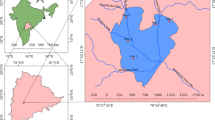
Chennai is the capital of the south Indian state of Tamil Nadu. According to the 2011 Indian census, the Chennai district had 7,088,000 inhabitants in an area of 426 km2 and the agglomeration area had 8.70 million inhabitants in 1189 km2, i.e. the fourth biggest in India (Directorate of Census Operations Tamil Nadu 2011). The city is located on the southeastern coast of India (13 °05′ N and 80 °18′ E), on a flat area known as the Eastern Coastal Plains with an average elevation of 6.7 m.a.s.l. and the highest point of 60 m.a.s.l. (Pulikesi et al. 2006).
The city has a tropical wet and dry climate (Köppen: Aw) with little changes in temperature throughout the year (Rajanikanth and Rajini Kanth 2020). Highest and lowest average monthly temperatures in the period 1971–2000 ranged from 28.9 to 37.1 °C and from 21.2 to 28.0 °C (Selvaraj et al. 2016). The highest temperatures usually correspond to late May and early June and the lowest to January (Prakash and Punyaseshudu 2015). The influence of the northeast monsoon provides a high average annual precipitation of around 1400 mm to Chennai, reaching 2570 mm in extreme years, like 2005 (Rajanikanth and Rajini Kanth 2020). Most of the rain takes place during the monsoon season, from mid-October to mid-December, and it is followed by a dry season (Selvaraj et al. 2016).
The two major rivers of the city are the Cooum River and the Adyar River. Both flow from west to east and drain into the Bay of Bengal. The rivers are connected by the Buckingham Canal, an artificial waterway that goes parallel to the coast (Mariappan 2014). The Pallikaranai catchment plays a relevant role because of its environmental value and the pressures that it is suffering. This catchment contains seven lakes, including the Pallikaranai marshland, a proposed Ramsar site (Shekhar 2020), and Sembakkam lake. The catchment was first comprised of vast agricultural lands and the Pallikaranai marshland before they were urbanized. Construction reduced the extension of the Pallikaranai marshland to only 6 km2 from around 50 km2 in former times (Vencatesan 2007; Steinbruch and Hörmann 2015; Sree Sharmila and Swathika 2016). In 2007, 3.17 km2 of the wetland was declared a Reserve Forest (Vencatesan 2007). However, there are two garbage-dump sites on its outskirts and in 2006, a sewage treatment plant was built near the marshland. Since then, the processed effluent is discharged into the water body (Steinbruch and Hörmann 2015).
Traditionally, the Sembakkam lake (12.9321° N, 80.1543° E) and other tanks in the catchment recharged its water during the monsoon season and provided water for irrigation during the dry season. Due to urbanization, the lake became ornamental and has suffered from degradation and abandonment. Currently, the lake is recharged with untreated sewage throughout the year. Until 2018, there was a landfill in the southwestern corner of the lake and still today, there are pumping groundwater that is later sold as drinking water. The lakes suffer from heavy pollution and eutrophication, making the water unsuitable for drinking, fishing, or recreational purposes because of high levels of total dissolved solids, biological oxygen demand, chemical oxygen demand, etc. (Raveen et al. 2008). Unfortunately, compromised contaminated water is not only an issue in Sembakkam Lake, but also in lakes, such as Rajakilpakkam, Madipakkam, and Medavakkam (Raveen et al. 2008).
In situ parameters, sampling and sample pre-treatment
Twenty-two sampling points (Table S1) were selected throughout Sembakkam Lake covering the whole lake area (Fig. 1). Samples of water and water hyacinth were collected in three sampling campaigns during the dry season (campaign 1, 04/07/2019; campaign 2, 07/08/2019; campaign 3, 04/09/2019). Sediments were collected in campaigns 1 and 3 and in an extra fourth campaign (20/09/2019) during the wet (monsoon) season, i.e. approximately every two months due to more stable concentrations compared to water. Due to the severe drought of 2019 and the subsequent reduction of the surface of the lake, some sampling points were unavailable in campaign 1.
After collection, water, sediment, and water hyacinth samples were transported in less than 3 h to the laboratories of the Civil Engineering Department of the India Institute of Technology Madras in a dark cooler (4 °C).
Some water quality parameters were measured in situ (pH, electrical conductivity, and temperature) at each sampling point with a WTW 3630 IDS portable multiparameter meter (Xylem Analytics, Germany). Water samples were collected in 250 ml polyethylene bottles previously washed with 10% HNO3 and Milli-Q water in the laboratory and rinsed three times with lake water afterwards. In the laboratory, water samples were filtered through 0.45 μm pore Teflon filters, acidified with HNO3 to pH 2 or lower, and stored at 4 °C until analysis. These methods are in accordance with ISO standard 5667 parts 1, 3, and 4 and with common procedures in scientific publications (Villa-Achupallas et al. 2018; Gemeda et al. 2021).
Sediment samples were collected with a Van Veen grab at each sampling point. Only grabs that showed adequate penetration were retained. The sediment material collected with five full grabs was collected and later mixed and homogenized to obtain a representative sample from each point that was stored in a dark cooler at 4 °C. In the laboratory, the samples were dried at 60 °C in an oven, disaggregated with an agate mortar, and sieved to fraction <63 μm (Jones and Turki 1997; Ouyang et al. 2002). Concentration of metals in sediments were referred to dry weight sediment (105 °C). These methods are in accordance with ISO standard 5667 parts 1, 12, and 15, and with common procedures in scientific publications (Rosado et al. 2015; Naifar et al. 2018).
Water hyacinth samples were collected in the Sembakkam lake manually from a boat in the locations where it was present. Once in the laboratory, plant samples were properly washed with Milli-Q water, and dried in an oven at 60 °C until constant weight. Dried samples were mechanically ground using a stainless-steel grinder (particle diameter of 100 µm). The resulting powder form of the plant sample was stored at room temperature until further analysis (Elmorsi et al. 2019).
Sediment and water hyacinth digestions
Sediment and water hyacinth samples underwent digestion in Teflon vessels in a PicoTrace® digestion block with 5 ml Suprapur nitric acid (HNO3) 65% (Merck, Germany) and 0.2 g of sediment or water hyacinth at 140 °C for 16 h. After the digestion phase, the block was cooled down to room temperature. The digestates were filtered, made up to 50 ml with Milli-Q water, and stored in polypropylene bottle in a fridge at 4 °C before the metal analysis. For quality control purposes, all digestion batches included a blank vessel with 5 ml of HNO3 only and a reference material vessel with reference soil SO-4 from the Canadian Certified Reference Materials Project. Recoveries were greater than 90% with the reference material for each metal and therefore considered satisfactory.
Sediments (1 g) underwent a extraction with 40 ml of 0.11 mol/L acetic acid prepared from Suprapur acetic acid at room temperature as described in the first step of the BCR-701 sequential extraction procedure (Pueyo et al. 2001) to obtain the acid extractable fraction, the most labile fraction of metals in sediments. After the extraction, the suspension was filtered and the filtrate was stored in a polypropylene bottle in a fridge at 4 °C prior to metal analysis. For quality control purposes, all extraction batches included a blank vessel with 40 ml of acetic acid only and a reference material vessel with reference material BCR-701 from the catalogue for certified reference materials of the European Commission's Joint Research Centre (JRC), achieving recoveries greater than 85% for all the elements and therefore considered acceptable.
Heavy metals measurements
In water samples as well as digestates of sediment and water hyacinth samples, Al, As, Cr, Cu, Fe, Mn, Ni, Pb, and Zn were measured using an ICP-OES (Thermo Scientific iCAP 6000) with axial and radial view.
Data analysis
The enrichment factor (EF) of heavy metals in sediments was calculated to assess the magnitude of enrichment and the potential anthropogenic involvement (Buat-Menard and Chesselet 1979; Aung et al. 2019). The following equation was employed: EF=(Csample/Alsample)/(Ccrust/Alcrust), in which Csample is the heavy metal concentration in the sample; Ccrust is the average heavy metal concentration in the upper continental crust according to Wedepohl (1995) in mg/kg: Al, 77440; As, 2; Cr, 35; Cu, 14.3; Fe, 30890; Mn, 728; Ni, 18.6; Pb, 17; Zn, 52; Alsample is the Al content in the sample; and Alcrust is the Al content in the continental crust (Wedepohl 1995). Aluminum was chosen as a normalization element due to its uniquely lithospheric origin (Thiombane et al. 2019). The EF was interpreted as follows, no enrichment (< 1), minor (1–3), moderate (3–5), moderately severe (5–10), severe (10–25), very severe (25–50), and extremely severe (> 50) (Amin et al. 2008; Rastegari Mehr et al. 2021).
The bioaccumulation factor (BF) was employed to show how different water hyacinth tissues bioaccumulate heavy metals. Thus, it was calculated as the ratio between the element concentrations across three plant tissues (root, stem, leaves) and those in its corresponding water: BF=Ctissue/Cwater (Carrillo-González and González-Chávez 2006; Chamba et al. 2017). The translocation factor (TF) was utilized to assess the transference of heavy metals from the roots to aerial parts (leaves and stem) in the water hyacinth. Therefore, TF was calculated as the ratio between the element concentrations in the aerial parts (stem, leaves) and those in the roots: TF = Caerial/Croots (Conesa et al. 2006; Chopin et al. 2008; Chamba et al. 2017). The BF and TF are relevant factors when assessing the phytoremediation capacity of a given species (Carrillo-González and González-Chávez 2006; Chopin et al. 2008).
R studio software version 1.4.1106 (R Development Core Team 2021) was used to carry out analysis of variance (ANOVA) with Tukey's post hoc test to check significant differences between temporal and spatial averages of heavy metals. Also, a Pearson correlation test was performed to look for heavy metals with similar behavior.
Results and discussion
In situ parameters
The average pH values recorded were 8.34 in campaign 1, 7.89 in campaign 2, and 8.46 in campaign 3. Hence, the pH was around neutral-alkaline. Although, the interval between averages can be considered moderate (0.45 pH units), the ANOVA test showed a statistically significant difference across sampling campaigns (p < 0.05). The Sembakkam lake receives a remarkable amount of sewage and wastewater that can influence the pH of the lake as well as the higher influence of rainwater throughout the year. These values fall within the range defined by the United States Environmental Protection Agency (USEPA) as suitable for aquatic life in the National Recommended Aquatic Life Criteria Table (USEPA 2021a) and by the Indian Central Pollution Control Board (CPCB) for the propagation of wildlife and fisheries (CPCB 1979; BIS 1992). Compared to other urban lakes in Chennai, the Sembakkam lake shows values below the Velachery lake (8.87) but above Perungudi (6.0), Karapakkam (6.1), Porur (6.2), Puzhal (7.0) and Nandhivaram (7.02) (Raji and Abraham 2018).
The average EC values recorded were 2864 µS/cm in campaign 1, 1666 µS/cm in campaign 2, and 1559 µS/cm in campaign 3. There is a decreasing trend in EC values from campaigns 1 to 3, which could be explained by the increasing rainfall recorded by the Chennai Metropolitan Water Supply and Sewerage Board (CMWSSB) during the year (CMWSSB 2020). The ANOVA test confirmed a statistically significant difference across sampling campaigns (p < 0.05). These values are above the general range defined by the USEPA (50–1500 µS/cm) for the conductivity of rivers in the United States (Esfahani et al. 2015; USEPA 2021b) and above the threshold value (1000 µS/cm) defined by CPCB for the propagation of wildlife and fisheries (CPCB 1979; BIS 1992). The average of sampling campaign 1 is also above the threshold for potable water (2500 µS/cm) of the potable water directive of the European Union (European Council 1998).
Mean temperature values recorded for the water in the lake were 32.8 °C in campaign 1, 27.9 °C in campaign 2, and 30.11°C in campaign 3. Temperatures also showed a significant difference across sampling campaigns (p < 0.05) according to the ANOVA test. Regarding dissolved oxygen, the values found were below 3 mg/L during campaign 1 (mean: 1.86 mg/L). To support aquatic life, they are considered “of concern” by the United States Environmental Protection Agency (USEPA 2021c) and below the adequate levels to grow Salmonids and Cyprinids by the European Union directive on the quality of fresh waters needing protection or improvement to support fish life (European Council 2006).
Water samples
The ranges of heavy metal concentrations found in the water of Sembakkam lake are presented in Table 1 and Fig. 2. The overall mean concentrations of heavy metals in water recorded in Sembakkam Lake were found to be Fe (11.89 µg/l) > Zn (5.55 µg/l) > Ni (5.47 µg/l) > Al (5.19 µg/l) > Cu (2.84 µg/l) > Mn (2.62 µg/l) > Pb (2.59 µg/l) > As (0.88 µg/l) > Cr (0.16 µg/l). The average concentrations for every campaign are shown in the supplementary material (Table S2). All the heavy metal concentrations recorded in this study were relatively low compared to other polluted lakes in India and around the world (Table 1).
Most of the metals studied were found harmless to the aquatic ecosystem when compared with the United Nations Economic Commission for Europe (UNECE) freshwater quality standards for the maintenance of aquatic life (Table 2). According to these guidelines, all measurements of As, Ni, and Zn are classified as Class I, indicating no anthropogenic pollution in the water (Fig. 3). However, some samples of Cr and most of the samples of Cu reach class II, which indicates that concentrations are below the midpoint between natural and chronically toxic levels. Finally, in the case of Pb, samples fell predominantly under class III and class IV, indicating concentrations above the midpoint between natural and chronically toxic levels and excursions beyond chronic criteria. Therefore, Pb represents the highest risk for the biota of the lake in terms of chronic toxic exposure. These concentrations are below the Indian thresholds for drinking water established by the Indian Bureau of Standards (BIS 2012).
Distribution (percentage) of the concentrations of heavy metals in the water of Sembakkam Lake, Chennai, India according to the classes defined in the UNECE guidelines for the maintenance of aquatic life (UNECE 1994)
Pb causes deleterious effects on blood and kidney of animals as well as on their reproductive, nervous, and immune systems (WHO 1989). Pb is known to bioaccumulate in organisms, mainly in biota consuming particulate matter, which is the case for benthic organisms. Hence they are the most likely to amass Pb to bioaccumulation up the food chain (WHO 2019).
Sembakkam Lake receives high input of untreated household effluents, predominantly on the south side. It is located next to an unused landfill but decades ago was surrounded by agricultural fields. Also, industrial effluents could be discharged into the lake. Cu could be attributed to the high input of sewage from the communities surrounding the lake (ATSDR 2002). In regards to Pb, domestic plumbing systems in which pipes, solder, and fittings contain Pb, including Polyvinyl chloride (PVC), which can lead to high Pb concentrations in the water due to leaching. Pb can also enter surface waters through the erosion of lead-containing soil particles and the disposal of waste containing Pb products (WHO 2019).
Heavy metal concentrations also exhibited significant spatial differences (p < 0.05) between northern and southern areas of the lake in the case of Cu, Ni, and Pb. The wastewater discharges could explain the higher concentrations located in the southwestern part of the lake.
Sediment samples
Total metal content
The ranges of heavy metal concentrations found in the sediments of Sembakkam lake are presented in Table 3. The overall mean concentrations of heavy metals recorded in Sembakkam Lake were found to be Al (36945 mg/kg) > Fe (26124 mg/kg) > Mn (323.30 mg/kg) > Cr (212.59 mg/kg) > Zn (168.03 mg/kg) > Cu (134.42 mg/kg) > Ni (73.58 mg/kg) > Pb (14.42 mg/kg) > As (2.94 mg/kg). Averages of every sampling campaign can be found in the supplementary material (Table S3). The heavy metal concentrations recorded in this study were in the range of other polluted lakes in India and around the world (Table 3). The concentrations for Al, Fe, and Mn are compatible with other results reported in a study conducted by Tholkappian et al. (2018) in Pulicat Lake, Chennai Coast of Tamil Nadu.
Regarding EF values, the most enriched metals were Cu and Cr, showing mostly severe enrichment, as can be seen in Fig. 4. Ni and Zn showed moderately severe enrichment. As, Fe, Mn, and Pb showed mostly minor enrichment. The high influx of untreated wastewater and leachates produced in the landfill nearby likely contributes to the enrichment, particularly in the case of Cu and Cr. Also, vestiges of pesticides containing Cu. Particularly for Cu, sewage sources can increase the Cu concentration in the sediment due to flocculation and binding to settling particles, or by complexation with natural ligands—e.g. humic substances (Leal et al. 1999). Other heavy metals, like As can also be enriched due to vestiges of pesticides containing As (Nikolaidis et al. 2004). In the case of Pb, in addition to the sources of domestic plumbing and others mentioned in the water section, the utilization of Pb in fuels in India until 2000 can explain a part of the enrichment of Pb in sediments.
Long et al. (1995) provides adverse biological effects guideline values for heavy metals in sediments in the form of three ranges defined by the effects range-low (ERL) and effects range-median (ERM). The concentrations below the ERL value represent a minimal-effects range; a range aimed to estimate conditions in which effects would be rarely observed. Concentrations equal to and above the ERL, but below the ERM, represent a possible-effects range within which effects would occasionally occur. The concentrations equivalent to and above the ERM value represent a probable-effects range within which effects would frequently occur. The values of the ERL (lower) and ERM (higher) in mg/kg in dry weight of sediment are the following (Long et al. 1995): As (8.20; 70), Cr (81; 370), Cu (34; 270), Ni (20.9; 51.6), Pb (46.7; 218) and Zn (150; 410).
When compared to adverse biological effects guideline values by Long et al. (1995), the concentrations for As, Pb and Zn were mostly below the ERL, which means that there is a probability of rare chronic toxic exposure to the biota of the lake. The concentrations for Cr and Cu were mostly in between the ERL and ERM values, meaning that there is a probability of occasional chronic toxic exposure to the biota of the lake. However, the concentrations recorded for Ni were mostly higher than the ERM values, meaning that Ni poses the highest risk for frequent chronic toxic exposure to the biota of the lake (Fig. 5).
Distribution (percentage) of the concentrations of heavy metals in the sediments of Sembakkam Lake, Chennai, India according to the effects range low (ERL) and the effects range median (ERM) intervals defined by Long et al. (1995)
Correlated metals suggest a similar source of pollution (Zhang et al. 2018). The results of the Pearson’s correlation matrix for concentrations in the Sembakkam lake sediment (Table 4) showed that across campaigns only Cu and Cr (r = 0.72), As and Fe (r = 0.70), and Ni and Cr (r = 0.94) had an r ≥ 0.7. These significant correlations suggest that they might be derived from untreated discharged sewage, leachates from the landfill, or other common sources.
Acid extractable fraction
The average labile fraction of heavy metals followed a decreasing order of Mn (43.69%) > As (15.63%) > Zn (13.24%) > Pb (4.78%) > Ni (3.43%) > Cu (1.51%) > Cr (0.13%) > Al (0.05%) > Fe (0.04%) in Sembakkam Lake. A box-plot representation can be found in Fig. 6. Averages of every sampling campaign can be found in the supplementary material (Table S3). The results obtained in this study show similarities with those found by Chandra Sekhar et al. (2004) regarding the high labile fraction of Zn, and with Wang et al. (2016) and El Nemr (2003) concerning the highest labile fraction for Mn. Chandra Sekhar et al. (2004) reported that Zn, Cu, and Pb were the most labile metals in the sediments of Kolleu Lake, India. The averages of the labile metal concentrations were found to be as follows: Zn > Cu > Pb > Cr > Ni. Wang et al. (2016) reported that the average labile fraction of metals in the sediments of Lake Taihu, China, followed Mn > Zn > Cu > Ni > Pb > Fe. El Nemr (2003) studied surface sediments of Lake Urullus, Egypt, and reported the labile fraction of metals in the lake in decreasing order of Mn > Cu > Ni > Pb > Zn > Cr.
An ANOVA test was conducted between the north and south areas of the lake. It confirmed that there was no significant spatial difference (p > 0.05) for metals Al, As, Cr, Cu, Fe, Ni, Pb, and Zn. Only in the case of Mn, a significant difference was found.
Water hyacinth samples
Regarding bioaccumulation factors, water hyacinth showed BF>1 values for all heavy metals, which implies that the plant has an efficient bioaccumulation system in the roots and can be considered an accumulator instead of an excluder (BF < 1) (Yanqun et al. 2005). BF values are shown in Fig. 7 and BF averages can be found in the supplementary material (Table S4). The average concentrations of heavy metals (mg/kg) in the tissues of the water hyacinth are also shown in the supplementary material (Table S4).
The highest BF values recorded in this study were for Al followed by Fe, Mn, Cr, Cu, Zn, Pb, Ni, and As (Fig. 7). These results suggest that water hyacinth does not absorb heavy metals homogeneously, and corroborate that absorption is not concentration-dependent. The high BF values of Mn, Fe, Cu, and Zn correspond to be essential plant macronutrients. Factors affecting heavy metal uptake and storage by aquatic plants could be either biological, e.g. species, age, and physiology, or non-biological (water physicochemical characteristics), e.g. temperature, salinity, and pH (Bonanno and Lo Giudice 2010). Specifically, heavy metal uptake by plants is influenced by heavy metal speciation such as humic complexes and free ions (Bonanno and Lo Giudice 2010).
The BF values of all metals were greater in the roots than in the aerial parts (stems and leaves) and they differ significantly according to the ANOVA and Tukey post hoc test results (p < 0.05). In the roots, the average BF of every metal can be grouped according to the degree of accumulation in millions of times for Al (2,285,239), hundreds of thousands of times for Fe (790,708), Mn (457,747) and Cr (353,451), tens of thousands of times for Cu (19,281), Zn (17,030) and Pb (11,227), and thousands of times for Ni (5162), and As (2,311). This implies a limited internal transport of heavy metals from the roots to the leaves and stem. Ephraim et al. (2018) confirmed the bioaccumulation potential of the water hyacinth finding BF>1 values and significant differences between the roots and the leaves for Ni, Cu, Pb and Zn. The mentioned author found the highest BF for Ni and the lowest for Zn. In a similar study, Eid et al. (2019) reported BF>1 values and significant differences in the roots compared to the leaves and stems for Mn, Fe, Cu, Zn, Cr, Pb, and Ni. The authors found the highest BF for Mn and the lowest for Ni. Other studies also proved the ability of water hyacinth to concentrate heavy metals in their roots (Kamari et al. 2017; Saha et al. 2017). The high capacity of the water hyacinth to store heavy metals in their roots could be contributing to a depuration process in the lake and a lower concentration of metals in the water.
Being an aquatic floating plant, water hyacinth absorbs heavy metals present in the water through the roots (Téllez et al. 2008). Physiological barriers against metal transport to the aerial parts are frequent in plants (Bose et al. 2008; Rahman et al. 2008; Saha et al. 2017). Metal binding proteins and the different biochemistry for accumulation between the root and stem could explain the absence of translocation to aerial parts (Lytle et al. 1998). Smaller and harder cations usually bind to the smaller atoms like N and O in the roots, while when transferred to the leaves and stems they bind to more complex composites such as oxalates and phytochelatins (Lytle et al. 1998).
The differences between roots and aerial tissues can also be seen in the translocation factor (TF) in Fig. 8. In the present study, all of the heavy metals recorded TF < 1 values, corroborating the water hyacinth’s low translocation capacity. Across all sampling campaigns, the highest average amount transferred from the roots to the leaves came from Zn (0.49) followed by Cu (0.30), Cr (0.26), Mn (0.14), Pb (0.12), As (0.11), Ni (0.11), Fe (0.04), and Al (0.04). The highest average amount transferred from the roots to the stems came from Zn (0.27) followed by Mn (0.25), Cu (0.24), Pb (0.12), Cr (0.11), As (0.11) Ni (0.11), Fe (0.04), and Al (0.03). All the TF averages are depicted in the supplementary material (Table S4).
In previous studies, Ephraim et al., (2018) recorded TF<1 values for Cu, Ni, and Pb, and only TF > 1 for Zn, Eid et al. (2019) reported TF < 1 values for Cr, Cu, Fe, Mn, Ni, and Zn, and only a TF>1 for Pb and Du et al. (2020) reported TF < 1 values for all analyzed metals in their study: Cu, Pb, and Zn. These results coincide with the present study regarding metals with TF < 1. However, they are in disagreement with the metals with TF > 1.
Transport from the roots to the leaves and stem was mostly in a uniform manner. Thus, most of the metals showed no significant difference (p > 0.05) between Stem-Leaves, with the exception of Mn, Zn, and Cr.
Conclusion
In this study, the heavy metal pollution in water, sediments, and water hyacinths of the Sembakkam lake was evaluated. It was found that Pb and, to a lesser extent, Cr and Cu in the water of the lake can pose a threat to biota due to toxicity. In sediments, Ni poses the highest risk of frequent chronic toxic exposure to biota while Cu and Cr pose a probability of occasional chronic toxic exposure. Also in sediments, Cu and Cr showed severe enrichment, Ni and Zn moderately severe enrichment and As, Fe, Mn, and Pb mostly minor enrichment. The severe and moderately severe enrichments could be explained by anthropogenic sources of pollution, like untreated wastewater as well as leachate produced in the closed dumpsite in the southwestern corner of the lake. Water hyacinths are abundant and their roots show concentrations up to hundreds of thousands of times higher than the water, contributing to a depuration of heavy metals. However, stems and leaves have concentrations significantly lower than the roots.
Proper management of sewage and waste is necessary to diminish the potential deleterious effects of metals on aquatic life and by extension, human health. Further research is needed to elucidate if the potential toxic effects identified in the Sembakkam lake are similar in other lakes of the catchment and the Pallikaranai catchment, and also in the fisheries and the surrounding groundwater that are consumed by local communities.
References
Abu El-Magd SA, Taha TH, Pienaar HH et al (2021) Assessing heavy metal pollution hazard in sediments of Lake Mariout. Egypt J African Earth Sci 176:104116. https://doi.org/10.1016/j.jafrearsci.2021.104116
Adelina C (2015) Internship Report IGCS. Chennai
Altindaǧ A, Yiǧit S (2005) Assessment of heavy metal concentrations in the food web of lake Beyşehir, Turkey. Chemosphere 60:552–556. https://doi.org/10.1016/j.chemosphere.2005.01.009
Amin B, Ismail A, Arshad A et al (2008) Anthropogenic impacts on heavy metal concentrations in the coastal sediments of Dumai, Indonesia. Environ Monit Assess 148:291–305. https://doi.org/10.1007/S10661-008-0159-Z
An YJ, Kampbell DH (2003) Total, dissolved, and bioavailable metals at Lake Texoma marinas. Environ Pollut 122:253–259. https://doi.org/10.1016/S0269-7491(02)00291-9
Arappor Iyakkam (2017) Why Chennai Stinks? Chennai
Arockia Vasanthi L, Revathi P, Mini J, Munuswamy N (2013) Integrated use of histological and ultrastructural biomarkers in Mugil cephalus for assessing heavy metal pollution in Ennore estuary, Chennai. Chemosphere 91:1156–1164. https://doi.org/10.1016/j.chemosphere.2013.01.021
ATSDR Agency for Toxic Substances and Disease Registry (2022) Toxicological profile for copper (draft for public comment). https://www.atsdr.cdc.gov/toxprofiles/tp132.pdf
Aung PP, Mao Y, Hu T et al (2019) Metal concentrations and pollution assessment in bottom sediments from Inle Lake Myanmar. J Geochemical Explor 207:106357. https://doi.org/10.1016/j.gexplo.2019.106357
BIS (1992) Indian standard water quality standards in India IS 2296:1992
BIS (2012) Indian standard drinking water specification IS 10500:2012
Bonanno G, Lo Giudice R (2010) Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol Indic 10:639–645. https://doi.org/10.1016/j.ecolind.2009.11.002
Bose S, Vedamati J, Rai V, Ramanathan AL (2008) Metal uptake and transport by Tyaha angustata L. grown on metal contaminated waste amended soil: An implication of phytoremediation. Geoderma 145:136–142. https://doi.org/10.1016/j.geoderma.2008.03.009
Buat-Menard P, Chesselet R (1979) Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Planet Sci Lett 42:399–411. https://doi.org/10.1016/0012-821X(79)90049-9
Carrillo-González R, González-Chávez MCA (2006) Metal accumulation in wild plants surrounding mining wastes. Environ Pollut 144:84–92. https://doi.org/10.1016/j.envpol.2006.01.006
Chamba I, Rosado D, Kalinhoff C et al (2017) Erato polymnioides – A novel Hg hyperaccumulator plant in Ecuadorian rainforest acid soils with potential of microbe-associated phytoremediation. Chemosphere 188:633–641. https://doi.org/10.1016/j.chemosphere.2017.08.160
Chandra Sekhar K, Chary NS, Kamala CT et al (2004) Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru lake by edible fish. Environ Int 29:1001–1008. https://doi.org/10.1016/S0160-4120(03)00094-1
Chaturvedi MC (2012) India’s waters: environment, economy, and development. CRC Press, Boca Raton
Chen R, Chen H, Song L et al (2019) Characterization and source apportionment of heavy metals in the sediments of Lake Tai (China) and its surrounding soils. Sci Total Environ 694:133819. https://doi.org/10.1016/j.scitotenv.2019.133819
Chopin EIB, Marin B, Mkoungafoko R et al (2008) Factors affecting distribution and mobility of trace elements (Cu, Pb, Zn) in a perennial grapevine (Vitis vinifera L.) in the Champagne region of France. Environ Pollut 156:1092–1098. https://doi.org/10.1016/j.envpol.2008.04.015
Ciazela J, Siepak M, Wojtowicz P (2018) Tracking heavy metal contamination in a complex river-oxbow lake system: middle Odra Valley, Germany/Poland. Sci Total Environ 616–617:996–1006. https://doi.org/10.1016/j.scitotenv.2017.10.219
CMWSSB (2020) Daily rainfall during 2019. http://123.63.203.150/dailyrain19.html. Accessed 28 May 2020
Conesa HM, Faz Á, Arnaldos R (2006) Heavy metal accumulation and tolerance in plants from mine tailings of the semiarid Cartagena–La Unión mining district (SE Spain). Sci Total Environ 366:1–11. https://doi.org/10.1016/j.scitotenv.2005.12.008
CPCB (1979) Surface water quality criteria for different uses
Das R, Chanakya HN, Rao L (2022) Study towards understanding foaming and foam stability in urban lakes. J Environ Manage 322:116111. https://doi.org/10.1016/J.JENVMAN.2022.116111
Devi NN, Sridharan B, Bindhu VM et al (2020) Investigation of role of retention storage in tanks (Small water bodies) on future urban flooding: a case study of Chennai city, India. Water (Switzerland). https://doi.org/10.3390/w12102875
Directorate of Census Operations Tamil Nadu (2011) Census of India 2011 - District Census Handbook Chennai, Part XII-B
Du Y, Wu Q, Kong D et al (2020) Accumulation and translocation of heavy metals in water hyacinth: maximising the use of green resources to remediate sites impacted by e-waste recycling activities. Ecol Indic 115:106384. https://doi.org/10.1016/j.ecolind.2020.106384
Eid EM, Shaltout KH, Moghanm FS et al (2019) Bioaccumulation and translocation of nine heavy metals by Eichhornia crassipes in Nile delta, Egypt: perspectives for phytoremediation. Int J Phytoremediation 21(8):821–830. https://doi.org/10.1080/15226514.2019.1566885
Eid EM, Galal TM, Sewelam NA et al (2020) Phytoremediation of heavy metals by four aquatic macrophytes and their potential use as contamination indicators: a comparative assessment. Environ Sci Pollut Res 27:12138–12151. https://doi.org/10.1007/s11356-020-07839-9
El Nemr A (2003) Assessment of heavy metal pollution in surface muddy sediments of lake Urullus, Southeastern Mediterranean, Egypt. Egypt J Aquat Biol Fish 7:67–90. https://doi.org/10.21608/ejabf.2003.1785
El-Khayat HMM, Abd-Elkawy S, Abou-Ouf NA et al (2018) Biochemical and histological assessment of some heavy metals on Biomphalaria alexandrina snails and Oreochromis niloticus fish in Lake Burullus, Egypt. Egypt J Aquat Biol Fish 12:159–182. https://doi.org/10.21608/ejabf.2018.9737
Elmorsi RR, Abou-El-Sherbini KS, Abdel-Hafiz Mostafa G, Hamed MA (2019) Distribution of essential heavy metals in the aquatic ecosystem of Lake Manzala, Egypt. Heliyon 5:e02276. https://doi.org/10.1016/J.HELIYON.2019.E02276
Ephraim BE, Ajayi IO, Ugbaja AN (2018) Pb, Zn, Cu, Ni and Co contents of water and sediments, in relation to phytoremediation and translocation by water hyacinth (Eichhornia crassipes Mart. Solms.) at some creeks of the great Kwa river, Southeastern Nigeria. Int J Env Pollut Res 6:16–37
Esfahani MR, Stretz HA, Wells MJM (2015) Abiotic reversible self-assembly of fulvic and humic acid aggregates in low electrolytic conductivity solutions by dynamic light scattering and zeta potential investigation. Sci Total Environ 537:81–92. https://doi.org/10.1016/j.scitotenv.2015.08.001
European Council (1998) Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1998:330:0032:0054:EN:PDF. Accessed 9 Apr 2017
European Council (2006) Directive 2006/44/EC of 6 September 2006 on the quality of fresh waters needing protection or improvement in order to support fish life. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006L0044&from=ES. Accessed 9 Apr 2017
Fraga RF, Rocha SMG, Lima Neto IE (2020) Impact of flow conditions on coliform dynamics in an urban lake in the Brazilian semiarid. Urban Water J 17:43–53. https://doi.org/10.1080/1573062X.2020.1734948
Gemeda FT, Guta DD, Wakjira FS, Gebresenbet G (2021) Occurrence of heavy metal in water, soil, and plants in fields irrigated with industrial wastewater in Sabata town, Ethiopia. Environ Sci Pollut Res 28:12382–12396. https://doi.org/10.1007/S11356-020-10621-6/TABLES/8
Haller L, Tonolla M, Zopfi J et al (2011) Composition of bacterial and archaeal communities in freshwater sediments with different contamination levels (Lake Geneva, Switzerland). Water Res 45:1213–1228. https://doi.org/10.1016/j.watres.2010.11.018
Hossain F, Degu AM, Woldemichael AT et al (2013) Water resources vulnerability in the context of rapid Urbanization of Dhaka City (a South Asian Megacity). Climate vulnerability: understanding and addressing threats to essential resources. Elsevier, Armsterdam, pp 393–404
Hussain SM, Anbalagan S, Kumar KS et al (2020) A baseline study on elemental concentration and potential ecological risk status of the surface sediments of Ashtamudi Lake, south west coast of India. Mar Pollut Bull 158:111410. https://doi.org/10.1016/j.marpolbul.2020.111410
International Monetary Fund (2019) World economic outlook: global manufacturing downturn. Rising Trade Barriers, Washington, DC
Jayaprakash M, Urban B, Velmurugan PM, Srinivasalu S (2010) Accumulation of total trace metals due to rapid urbanization in microtidal zone of Pallikaranai marsh, South of Chennai, India. Environ Monit Assess 170:609–629. https://doi.org/10.1007/s10661-009-1261-6
Jayaraman N (2019) Viewpoint: why India’s Chennai has run out of water. BBC News
Ji Z, Zhang H, Zhang Y et al (2019) Distribution, ecological risk and source identification of heavy metals in sediments from the Baiyangdian Lake, Northern China. Chemosphere 237:124425. https://doi.org/10.1016/j.chemosphere.2019.124425
Jiang Q, Liu M, Wang J, Liu F (2018) Feasibility of using visible and near-infrared reflectance spectroscopy to monitor heavy metal contaminants in urban lake sediment. Catena 162:72–79. https://doi.org/10.1016/J.CATENA.2017.11.020
Jones B, Turki A (1997) Distribution and speciation of heavy metals in surficial sediments from the Tees Estuary, north-east England. Mar Pollut Bull 34:768–779. https://doi.org/10.1016/S0025-326X(97)00047-7
Kamari A, Yusof N, Abdullah H et al (2017) Assessment of heavy metals in water, sediment, Anabas testudineus and Eichhornia crassipes in a former mining pond in Perak Malaysia. Chem Ecol 33(7):637–651. https://doi.org/10.1080/02757540.2017.1351553
Karthikeyan P, Vennila G, Nanthakumar G, Aswini M (2020) Dataset for spatial distribution and pollution indices of heavy metals in the surface sediments of Emerald Lake, Tamil Nadu, India. Data Br 28:104877. https://doi.org/10.1016/j.dib.2019.104877
Kelkar U, Balachandra P, Gurtoo A (2011) Assessing indian cities for vulnerability to climate change. In: 2nd International conference on environvironment science development, Vol 4, pp 246–250
Kumar MD, Tortajada C (2020) Introduction. In: Kumar MD, Tortajada C (eds) Assessing wastewater management in India. Springer, Singapore, pp 1–5
Kumar R, Parvaze S, Huda MB, Allaie SP (2022) The changing water quality of lakes—a case study of Dal Lake, Kashmir Valley. Environ Monit Assess 194:1–16. https://doi.org/10.1007/S10661-022-09869-X/TABLES/1
Lakshmi DMSR, Thulasi MD, Ahmad TJ (2018) Geo-chemical evaluation of heavy metals in the lakes of south Chennai, Tamil Nadu, India. Int J Res Soc Sci 8:643–657
Lan T, Almered Olson E, Alpokay S (2014) Environmental stresses and resource use in coastal urban and Peri-Urban Regions DPSIR Approach to SECOA’s 17 case studies. Sapienza Universitá Editrice, Rome
Leal MFC, Vasconcelos MTSD, Van Den Berg CMG (1999) Copper-induced release of complexing ligands similar to thiols by Emiliania huxleyi in seawater cultures. Limnol Oceanogr 44:1750–1762. https://doi.org/10.4319/lo.1999.44.7.1750
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manage 19:81–97. https://doi.org/10.1007/BF02472006
Lytle CM, Lytle PW, Yang N et al (1998) Reduction of Cr(VI) to Cr(III) by wetland plants: potential for in situ heavy metal detoxification. Environ Sci Technol 32:3087–3093. https://doi.org/10.1021/es980089x
Mariappan J (2014) Public consultation for Adyar river restoration to be held in Chennai on Wednesday. The Times of India
Massuel S, Perrin J, Mascre C et al (2014) Managed aquifer recharge in South India: what to expect from small percolation tanks in hard rock? J Hydrol 512:157–167. https://doi.org/10.1016/j.jhydrol.2014.02.062
Mwita JC (2011) Determination of heavy metal content in water, sediment and microalgae from Lake Victoria East Africa. Open Environ Eng J 4:156–161. https://doi.org/10.2174/1874829501104010156
Naifar I, Pereira F, Zmemla R et al (2018) Spatial distribution and contamination assessment of heavy metals in marine sediments of the southern coast of Sfax, Gabes Gulf, Tunisia. Mar Pollut Bull 131:53–62. https://doi.org/10.1016/J.MARPOLBUL.2018.03.048
Newete SW, Erasmus BFN, Weiersbye IM, Byrne MJ (2016) Sequestration of precious and pollutant metals in biomass of cultured water hyacinth (Eichhornia crassipes). Environ Sci Pollut Res 23:20805–20818. https://doi.org/10.1007/s11356-016-7292-y
Nikolaidis NP, Dobbs GM, Chen J, Lackovic JA (2004) Arsenic mobility in contaminated lake sediments. Environ Pollut 129:479–487. https://doi.org/10.1016/j.envpol.2003.11.005
Ochieng EZ, Lalah JO, Wandiga SO (2006) Heavy metals in water and surface sediments in Winam gulf of lake Victoria, Kenya. Bull Environ Contam Toxicol 77:459–468. https://doi.org/10.1007/s00128-006-1087-7
Ouyang Y, Higman J, Thompson J et al (2002) Characterization and spatial distribution of heavy metals in sediment from Cedar and Ortega rivers subbasin. J Contam Hydrol 54:19–35. https://doi.org/10.1016/S0169-7722(01)00162-0
Öztürk M, Özözen G, Minareci O, Minareci E (2008) Determination of heavy metals in of fishes, water and sediment from the Demirköprü Dam Lake (Turkey). J Appl Biol Sci 2:99–104
Palanisami K, Jegadeesan M, Fujita K, Kono Y (2008) Impacts of the tank modernization programme on tank performance in Tamil Nadu State. Kyoto, India
Pistocchi A, Udias A, Grizzetti B et al (2017) An integrated assessment framework for the analysis of multiple pressures in aquatic ecosystems and the appraisal of management options. Sci Total Environ 575:1477–1488. https://doi.org/10.1016/j.scitotenv.2016.10.020
Prabhu B, Batvari D, Surendran A (2015) Assessment of heavy metal contamination in Chemberambakkam lake water, Chennai, Tamil Nadu. J Chem Pharm Res, India, p 7
Prakash TJ, Punyaseshudu D (2015) Analysis of tropical cyclone and its impact on coastal areas. Int J Innov Eng Technol 5:95–105
Prasanna MV, Praveena SM, Chidambaram S et al (2012) Evaluation of water quality pollution indices for heavy metal contamination monitoring: a case study from Curtin Lake, Miri City, East Malaysia. Environ Earth Sci 67:1987–2001. https://doi.org/10.1007/S12665-012-1639-6/FIGURES/5
Pueyo M, Rauret G, Lück D et al (2001) Certification of the extractable contents of Cd, Cr, Cu, Ni, Pb and Zn in a freshwater sediment following a collaboratively tested and optimised three-step sequential extraction procedure. J Environ Monit 3:243–250. https://doi.org/10.1039/b010235k
Pulikesi M, Baskaralingam P, Elango D et al (2006) Air quality monitoring in Chennai, India, in the summer of 2005. J Hazard Mater 136:589–596. https://doi.org/10.1016/j.jhazmat.2005.12.039
R Development Core Team (2021) No Title. https://www.R-project.org/
Rahman MA, Hasegawa H, Ueda K et al (2008) Arsenic uptake by aquatic macrophyte Spirodela polyrhiza L.: interactions with phosphate and iron. J Hazard Mater 160:356–361. https://doi.org/10.1016/j.jhazmat.2008.03.022
Rajanikanth J, Rajini Kanth TV (2020) Chennai weather data analysis using hybrid data mining techniques. Springer, Cham, pp 357–367
Rajeshkumar S, Liu Y, Zhang X et al (2018) Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 191:626–638. https://doi.org/10.1016/j.chemosphere.2017.10.078
Raji PK, Abraham M (2018) Comparative study of water quality of different lakes in Chennai. Rasayan J Chem 11:828–833. https://doi.org/10.7324/RJC.2018.1122005
Rastegari Mehr M, Shakeri A, Amjadian K et al (2021) Bioavailability, distribution and health risk assessment of arsenic and heavy metals (HMs) in agricultural soils of Kermanshah Province, west of Iran. J Environ Heal Sci Eng 19:107–120. https://doi.org/10.1007/S40201-020-00585-7/TABLES/6
Raveen R, Chennakrishnan C, Stephen A, Nadu T (2008) Impact of pollution on the quality of water in three freshwater lakes of suburban Chennai. Nat Environ Pollut Technol 7:61–64
Reddy MV, Babu KS, Balaram V, Satyanarayanan M (2012) Assessment of the effects of municipal sewage, immersed idols and boating on the heavy metal and other elemental pollution of surface water of the eutrophic Hussainsagar Lake (Hyderabad, India). Environ Monit Assess 184:1991–2000. https://doi.org/10.1007/s10661-011-2094-7
Rosado D, Usero J, Morillo J (2015) Application of a new integrated sediment quality assessment method to Huelva estuary and its littoral of influence (Southwestern Spain). Mar Pollut Bull 98:106–14. https://doi.org/10.1016/j.marpolbul.2015.07.008
Saha P, Shinde O, Sarkar S (2017) Phytoremediation of industrial mines wastewater using water hyacinth. Int J Phytoremediation 19(1):87–96. https://doi.org/10.1080/15226514.2016.1216078
Santhiya G, Lakshumanan C, Jonathan MP et al (2011) Metal enrichment in beach sediments from Chennai Metropolis, SE coast of India. Mar Pollut Bull 62:2537–2542. https://doi.org/10.1016/j.marpolbul.2011.08.019
Selvaraj K, Pandiyan J, Yoganandan V, Agoramoorthy G (2016) India contemplates climate change concerns after floods ravaged the coastal city of Chennai. Ocean Coast Manag 129:10–14. https://doi.org/10.1016/j.ocecoaman.2016.04.017
Sharawat I, Dahiya R, Dahiya RP et al (2019) Policy options for managing the water resources in rapidly expanding cities: a system dynamics approach. Sustain Water Resour Manag 5:1201–1215. https://doi.org/10.1007/s40899-018-0296-7
Sharma B, Gulati A, Mohan G, et al (2018) Water productivity mapping of major Indian crops
Shekhar L (2020) How an international tag can help save the Pallikaranai marshland. In: Citiz. Matters. https://chennai.citizenmatters.in/how-international-tag-can-help-save-pallikarnai-marshland-14057. Accessed 28 Jul 2020
Sree Sharmila T, Swathika R (2016) Prediction of environmental changes in dumpyard sites: A case study of pallikaranai dumpyard, Chennai, Tamil Nadu. In: Communications in computer and information science. Springer, Berlin, pp 674–682
Steinbruch F, Hörmann G (2015) Towards integrated water management of Pallikaranai wetland in Chennai City. Kiel, India
Téllez TR, de López EMR, Granado GL et al (2008) The water hyacinth, Eichhornia crassipes: an invasive plant in the Guadiana River Basin (Spain). Aquat Invasions 3:42–53. https://doi.org/10.3391/ai.2008.3.1.8
Thiombane M, Di Bonito M, Albanese S et al (2019) Geogenic versus anthropogenic behaviour and geochemical footprint of Al, Na, K and P in the Campania region (Southern Italy) soils through compositional data analysis and enrichment factor. Geoderma 335:12–26. https://doi.org/10.1016/J.GEODERMA.2018.08.008
Tholkappian M, Ravisankar R, Chandrasekaran A et al (2018) Assessing heavy metal toxicity in sediments of Chennai Coast of Tamil Nadu using energy dispersive x-ray fluorescence spectroscopy (EDXRF) with statistical approach. Toxicol Reports 5:173–182. https://doi.org/10.1016/j.toxrep.2017.12.020
Udias A, Pistocchi A, Vigiak O et al (2020) ESPRES: a web application for interactive analysis of multiple pressures in aquatic ecosystems. Sci Total Environ 744:140792. https://doi.org/10.1016/j.scitotenv.2020.140792
UNECE United Nations Economic Commission for Europe (1994) Standard Statistical Classification of Surface Freshwater Quality for the Maintenance of Aquatic Life. In: Readings in International Environment Statistics. United Nations Economic Commission for Europe, United Nations, New York and Geneva
United Nations (2015) Transforming our world: the 2030 Agenda for Sustainable Development
USEPA (2021a) National Recommended Water Quality Criteria - Aquatic Life Criteria Table. https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table. Accessed 17 Jul 2021a
USEPA (2021b) Website of the United States Environmental Protection Agency. https://archive.epa.gov/water/archive/web/html/vms59.html. Accessed 17 Jul 2021b
USEPA (2021c) Indicators: Dissolved Oxygen. https://www.epa.gov/national-aquatic-resource-surveys/indicators-dissolved-oxygen. Accessed 9 Oct 2021c
Vasanthi LA, Revathi P, Babu Rajendran R, Munuswamy N (2017) Detection of metal induced cytopathological alterations and DNA damage in the gills and hepatopancreas of green mussel Perna viridis from Ennore Estuary, Chennai, India. Mar Pollut Bull 117:41–49. https://doi.org/10.1016/j.marpolbul.2017.01.040
Vencatesan J (2007) Protecting wetlands. Curr Sci 93:288–290
Villa-Achupallas M, Rosado D, Aguilar S, Galindo-Riaño MD (2018) Water quality in the tropical Andes hotspot: the Yacuambi river (southeastern Ecuador). Sci Total Environ 633:50–58. https://doi.org/10.1016/j.scitotenv.2018.03.165
Wang D, Gong M, Li Y et al (2016) In situ, high-resolution profiles of labile metals in sediments of Lake Taihu. Int J Environ Res Public Health 13:884. https://doi.org/10.3390/ijerph13090884
(2019) Water crisis in Chennai: why Chennai’s water crisis should worry you. Times of India
Wedepohl KH (1995) The composition of the continental crust. Geochim Cosmochim Acta 59:1217–1232. https://doi.org/10.1016/0016-7037(95)00038-2
WHO World Health Organization (1989) Lead: environmental aspects-environmental health criteria 85
WHO World Health Organization (2019) Preventing disease through healthy environments: exposure to lead: a major public health concern. World Health Organization
Yanqun Z, Yuan L, Jianjun C et al (2005) Hyperaccumulation of Pb, Zn and Cd in herbaceous grown on lead-zinc mining area in Yunnan, China. Environ Int 31:755–762. https://doi.org/10.1016/j.envint.2005.02.004
Yazidi A, Saidi S, Ben Mbarek N, Darragi F (2017) Contribution of GIS to evaluate surface water pollution by heavy metals: case of Ichkeul Lake (Northern Tunisia). J African Earth Sci 134:166–173. https://doi.org/10.1016/j.jafrearsci.2017.06.009
Zhang C, Shan B, Zhao Y et al (2018) Spatial distribution, fractionation, toxicity and risk assessment of surface sediments from the Baiyangdian Lake in northern China. Ecol Indic 90:633–642. https://doi.org/10.1016/j.ecolind.2018.03.078
Acknowledgements
The authors thank the financial support of the Deutsche Hydrologische Gesellschaft (DHG).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose
Additional information
Editorial responsibility: Maryam Shabani.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rosado, D., Castillo, F., Nambi, I. et al. Evaluating heavy metal levels and their toxicity risks in an urban lake in Chennai, India. Int. J. Environ. Sci. Technol. 21, 1849–1864 (2024). https://doi.org/10.1007/s13762-023-05086-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05086-2