Abstract
BACKGROUND:
Different methods have been used to inject stem cells into the eye for research. We previously explored the intravitreal route. Here, we investigate the efficacy of intravenous and subretinal-transplanted human dental pulp stem cells (DPSCs) in rescuing the photoreceptors of a sodium iodate-induced retinal degeneration model.
Methods:
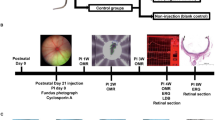
Three groups of Sprague Dawley rats were used: intervention, vehicle group and negative control groups (n = 6 in each). Intravenous injection of 60 mg/kg sodium iodate (day 0) induced retinal degeneration. On day 4 post-injection of sodium iodate, the rats in the intervention group received intravenous DPSC and subretinal DPSC in the right eye; rats in the vehicle group received subretinal Hank’s balance salt solution and intravenous normal saline; while negative control group received nothing. Electroretinogram (ERG) was performed to assess the retinal function at day 0 (baseline), day 4, day 11, day 18, day 26, and day 32. By the end of the study at day 32, the rats were euthanized, and both their enucleated eyes were sent for histology.
Results:
No significant difference in maximal ERG a-wave (p = 0.107) and b-wave, (p = 0.153) amplitude was seen amongst the experimental groups. However, photopic 30 Hz flicker amplitude of the study eye showed significant differences in the 3 groups (p = 0.032). Within the intervention group, there was an improvement in 30 Hz flicker ERG response of all 6 treated right eyes, which was injected with subretinal DPSC; while the 30 Hz flicker ERG of the non-treated left eyes remained flat. Histology showed improved outer nuclear layer thickness in intervention group; however, findings were not significant compared to the negative and vehicle groups.
Conclusion:
Combination of subretinal and intravenous injection of DPSCs may have potential to rescue cone function from a NaIO3-induced retinal injury model.








Similar content being viewed by others
References
Leow SN, Luu CD, Hairul Nizam MH, Mok PL, Ruhaslizan R, Wong HS, et al. Safety and efficacy of human Wharton’s Jelly-derived mesenchymal stem cells therapy for retinal degeneration. PLoS One. 2015;10:e0128973.
Jiang Y, Zhang Y, Zhang L, Wang M, Zhang X, Li X. Therapeutic effect of bone marrow mesenchymal stem cells on laser-induced retinal injury in mice. Int J Mol Sci. 2014;15:9372–85.
Machalińska A, Rogińska D, Pius-Sadowska E, Kawa MP, Paczkowska E, Rudnicki M, et al. Neuroprotective and antiapoptotic activity of lineage-negative bone marrow cells after intravitreal injection in a mouse model of acute retinal injury. Stem Cells Int. 2015;2015:620364.
Zhang Y, Wang W. Effects of bone marrow mesenchymal stem cell transplantation on light-damaged retina. Invest Ophthalmol Vis Sci. 2010;51:3742–8.
Haddad-Mashadrizeh A, Bahrami AR, Matin MM, Edalatmanesh MA, Zomorodipour A, Gardaneh M, et al. Human adipose-derived mesenchymal stem cells can survive and integrate into the adult rat eye following xenotransplantation. Xenotransplantation. 2013;20:165–76.
Hambright D, Park KY, Brooks M, McKay R, Swaroop A, Nasonkin IO. Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Mol Vis. 2012;18:920–36.
Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, Yao J, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One. 2011;6:e18992.
Alsaeedi HA, Koh AE, Lam C, Rashid MBA, Harun MHN, Saleh MFBM, et al. Dental pulp stem cells therapy overcome photoreceptor cell death and protects the retina in a rat model of sodium iodate-induced retinal degeneration. J Photochem Photobiol B. 2019;198:111561.
Banin E, Obolensky A, Idelson M, Hemo I, Reinhardtz E, Pikarsky E, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells. 2006;24:246–57.
Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251–5.
Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Concise review: dental pulp stem cells: a novel cell therapy for retinal and central nervous system repair. Stem Cells. 2017;35:61–7.
Kawashima N. Characterisation of dental pulp stem cells: a new horizon for tissue regeneration? Arch Oral Biol. 2012;57:1439–58.
Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80–90.
Leong WK, Henshall TL, Arthur A, Kremer KL, Lewis MD, Helps SC, et al. Human adult dental pulp stem cells enhance post stroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl Med. 2012;1:177–87.
Fang CZ, Yang YJ, Wang QH, Yao Y, Zhang XY, He XH. Intraventricular injection of human dental pulp stem cells improves hypoxic-ischemic brain damage in neonatal rats. PLoS One. 2013;8:e66748.
Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013;54:7544–56.
Mead B, Hill LJ, Blanch RJ, Ward K, Logan A, Berry M, et al. Mesenchymal stromal cell-mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy. 2016;18:487–96.
Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13–27.
Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH, et al. A fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–9.
Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e109305.
Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238:120–32.
Harper MM, Grozdanic SD, Blits B, Kuehn MH, Zamzow D, Buss JE, et al. Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Invest Ophthalmol Vis Sci. 2011;52:4506–15.
Bray AF, Cevallos RR, Gazarian K, Lamas M. Human dental pulp stem cells respond to cues from the rat retina and differentiate to express the retinal neuronal marker rhodopsin. Neuroscience. 2014;280:142–55.
Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4:303–6.
Qu L, Gao L, Xu H, Duan P, Zeng Y, Liu Y, et al. Combined transplantation of human mesenchymal stem cells and human retinal progenitor cells into the subretinal space of RCS rats. Sci Rep. 2017;7:199.
Strauss O, Stumpff F, Mergler S, Wienrich M, Wiederholt M. The Royal College of Surgeons rat: an animal model for inherited retinal degeneration with a still unknown genetic defect. Acta Anat (Basel). 1998;162:101–11.
Balmer J, Zulliger R, Roberti S, Enzmann V. Retinal cell death caused by sodium iodate involves multiple caspase-dependent and caspase-independent cell-death pathways. Int J Mol Sci. 2015;16:15086–103.
Koh AE, Alsaeedi HA, Rashid MBA, Lam C, Harun MHN, Saleh MFBM, et al. Retinal degeneration rat model: A study on the structural and functional changes in the retina following injection of sodium iodate. J Photochem Photobiol B. 2019;196:111514.
Traverse JH. Is there a role for intravenous stem cell delivery in nonischemic cardiomyopathy? Circ Res. 2017;120:256–8.
Bakondi B, Girman S, Lu B, Wang S. Multimodal delivery of isogenic mesenchymal stem cells yields synergistic protection from retinal degeneration and vision loss. Stem Cells Transl Med. 2017;6:444–57.
Xian B, Huang B. The immune response of stem cells in subretinal transplantation. Stem Cell Res Ther. 2015;6:161.
Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–61.
Pan D, Chang X, Xu M, Zhang M, Zhang S, Wang Y, et al. UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. J Chem Neuroanat. 2019;96:134–9.
Tu HY, Watanabe T, Shirai H, Yamasaki S, Kinoshita M, Matsushita K, et al. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine. 2019;39:562–74.
Guan Y, Cui L, Qu Z, Lu L, Wang F, Wu Y, et al. Subretinal transplantation of rat MSCs and erythropoietin gene modified rat MSCs for protecting and rescuing degenerative retina in rats. Curr Mol Med. 2013;13:1419–31.
Sothilingam V, Mühlfriedel R, Tanimoto N, Seeliger MW. In-depth functional analysis of rodents by full-field electroretinography. Methods Mol Biol. 2018;1715:207–13.
Ball SL, Petry HM. Noninvasive assessment of retinal function in rats using multifocal electroretinography. Invest Ophthalmol Vis Sci. 2000;41:610–7.
Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2051–9.
Girman SV, Wang S, Lund RD. Time course of deterioration of rod and cone function in RCS rat and the effects of subretinal cell grafting: a light- and dark-adaptation study. Vision Res. 2005;45:343–54.
Yang Y, Ng TK, Ye C, Yip YW, Law K, Chan SO, et al. Assessing sodium iodate–induced outer retinal changes in rats using confocal scanning laser ophthalmoscopy and optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:1696–705.
Machalińska A, Lejkowska R, Duchnik M, Kawa M, Rogińska D, Wiszniewska B, et al. Dose-dependent retinal changes following sodium iodate administration: application of spectral-domain optical coherence tomography for monitoring of retinal injury and endogenous regeneration. Curr Eye Res. 2014;39:1033–41.
Wang J, Iacovelli J, Spencer C, Saint-Geniez M. Direct effect of sodium iodate on neurosensory retina. Invest Ophthalmol Vis Sci. 2014;55:1941–53.
Phillips MJ, Wallace KA, Dickerson SJ, Miller MJ, Verhoeven AD, Martin JM, et al. Blood-derived human iPS cells generate optic vesicle–like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci. 2012;53:2007–19.
Zhang X, Zhou Y, Li H, Wang R, Yang D, Li B, et al. Intravenous administration of DPSCs and BDNF improves neurological performance in rats with focal cerebral ischemia. Int J Mol Med. 2018;41:3185–94.
Machalińska A, Lubiński W, Kłos P, Kawa M, Baumert B, Penkala K, et al. Sodium iodate selectively injuries the posterior pole of the retina in a dose-dependent manner: morphological and electrophysiological study. Neurochem Res. 2010;35:1819–27.
Yamashita H, Yamasaki K, Sugihara K, Miyata H, Tsutsumi S, Iwaki Y. Full-field electroretinography obtained using a contact lens electrode with built-in high-intensity white-light-emitting diodes can be utilized in toxicological assessments in rats. Ophthalmic Res. 2009;42:15–20.
Acknowledgement
The authors would like to acknowledge the funding provided by UKM through the Fundamental Grant awarded to Drs Mae-Lynn Catherine Bastion and Lam Chen Shen (Project Code FF 2017-096) and the Publication Grant awarded to Dr Mae-Lynn Catherine Bastion (Project Code GP-K009894). The authors also extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number 375213500. Last but not least, the authors acknowledge CryoCord Sdn Bhd for providing the DPSC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
The ethics approval was obtained from UKM Animal Ethical Committee (Reference code: UKM FPR.4/244/FF-2017-096).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
About this article
Cite this article
Lam, C., Alsaeedi, H.A., Koh, A.EH. et al. Human Dental Pulp Stem Cells (DPSCs) Therapy in Rescuing Photoreceptors and Establishing a Sodium Iodate-Induced Retinal Degeneration Rat Model. Tissue Eng Regen Med 18, 143–154 (2021). https://doi.org/10.1007/s13770-020-00312-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00312-1