Abstract
Purpose
To investigate the prevalence of neuro-functional disability and its determinants 12 months after community-acquired bacterial meningitis (CABM) in adult patients.
Methods
In a prospective multicenter cohort study (COMBAT), all consecutive cases of CABM were enrolled and followed up for 12 months. Neuro-functional disability at 12 months was evaluated using a combination of the Glasgow Outcome Scale (functional disability), and the modified Rankin Disability Scale (physical disability). Factors associated with neuro-functional disability were identified by multivariate logistic regression.
Results
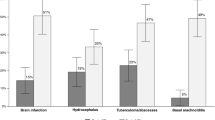
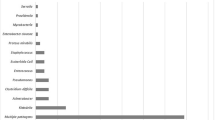
Among 281 patients, 84 (29.9%) patients exhibited neuro-functional disability at 12 months: 79 (28.1%) with functional disability and 51 (18.1%) with physical disability. Overall, 6 patients (2.1%) died during the follow-up. The most common pathogen identified was Streptococcus pneumoniae (131/272, 48.2%); 77/268 patients (28.7%) had a physical disability at hospital discharge. Factors independently associated with 12-month neuro-functional disability were a pneumococcal meningitis (adjusted OR = 2.8; 95% confidence interval (CI) = [1.3; 6.7]), the presence of a physical disability at hospital discharge (aOR = 2.3; 95%CI = [1.2; 4.4]) and the presence of behavioral disorders at hospital-discharge (aOR = 5.9; 95%CI = [1.6; 28.4]). Dexamethasone use was not significantly associated with neuro-functional disability (OR = 0.2; 95%CI = [< 0.1;1.3]).
Conclusion
Neuro-functional disability is frequently reported 12 months after CABM. Detailed neurological examination at discharge is needed to improve the follow-up.
Trial registration
NCT01730690.

Similar content being viewed by others
Availability of data and material
Data will be made upon reasonable request to the corresponding author.
Code availability
Not applicable.
References
van de Beek D, Brouwer MC, Koedel U, Wall EC. Community-acquired bacterial meningitis. The Lancet. 2021;398:1171–83. https://doi.org/10.1016/S0140-6736(21)00883-7.
Bijlsma MW, Brouwer MC, Kasanmoentalib ES, Kloek AT, Lucas MJ, Tanck MW, et al. Community-acquired bacterial meningitis in adults in the Netherlands, 2006–14: a prospective cohort study. Lancet Infect Dis. 2016;16:339–47. https://doi.org/10.1016/S1473-3099(15)00430-2.
Lucas MJ, Brouwer MC, van de Beek D. Neurological sequelae of bacterial meningitis. J Infect. 2016;73:18–27. https://doi.org/10.1016/j.jinf.2016.04.009.
Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–4. https://doi.org/10.1016/s0140-6736(75)92830-5.
Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15:573–85. https://doi.org/10.1089/neu.1998.15.573.
Fayol P, Carrière H, Habonimana D, Preux P-M, Dumond J-J. French version of structured interviews for the Glasgow Outcome Scale: guidelines and first studies of validation. Ann Readapt Med Phys. 2004;47:142–56. https://doi.org/10.1016/j.annrmp.2004.01.004.
Rankin J. Cerebral vascular accidents in patients over the age of 60. II Prognosis Scott Med J. 1957;2:200–15. https://doi.org/10.1177/003693305700200504.
United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: interim results. UK-TIA Study Group. Br Med J (Clin Res Ed) 1988;296:316–20.
des Portes V. Long-term follow-up of bacterial meningitis—sequels in children and adults: incidence, type, and assessment issues. Med Mal Infect. 2009;39:572–80. https://doi.org/10.1016/j.medmal.2009.02.019.
van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849–59. https://doi.org/10.1056/NEJMoa040845.
Kastenbauer S, Pfister H-W. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain. 2003;126:1015–25. https://doi.org/10.1093/brain/awg113.
Muralidharan R, Mateen FJ, Rabinstein AA. Outcome of fulminant bacterial meningitis in adult patients. Eur J Neurol. 2014;21:447–53. https://doi.org/10.1111/ene.12328.
Tubiana S, Varon E, Biron C, Ploy M-C, Mourvillier B, Taha M-K, et al. Community-acquired bacterial meningitis in adults: in-hospital prognosis, long-term disability and determinants of outcome in a multicentre prospective cohort. Clin Microbiol Infect. 2020;26:1192–200. https://doi.org/10.1016/j.cmi.2019.12.020.
Nguyen THM, Tran THC, Thwaites G, Ly VC, Dinh XS, Ho Dang TN, et al. Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis. N Engl J Med. 2007;357:2431–40. https://doi.org/10.1056/NEJMoa070852.
van de Beek D, Cabellos C, Dzupova O, Esposito S, Klein M, Kloek AT, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22 Suppl 3:S37-62. https://doi.org/10.1016/j.cmi.2016.01.007.
Weir J, Steyerberg EW, Butcher I, Lu J, Lingsma HF, McHugh GS, et al. Does the extended Glasgow Outcome Scale add value to the conventional Glasgow Outcome Scale? J Neurotrauma. 2012;29:53–8. https://doi.org/10.1089/neu.2011.2137.
Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981;44:285–93. https://doi.org/10.1136/jnnp.44.4.285.
Acknowledgements
Combat study group; Principal investigator: X. Duval. Steering Committee: B. Hoen, B. Mourvillier, E. Varon, S. Tubiana, M. C. Ploy. Scientific committee: steering committee and the following members F. Caron, Pe. Bollaert, O. Gaillot, Mk. Taha, C. Poyart, S Bonacorsi, F. Vandenesch, E. Cambau, M Lecuit, A. Gravet, B. Frachet, T. Dde Broucker, D. Levy Bruhl, F. Raffi. Clinical centers: B. Abraham, F. Ader, E. Ancel, N. Anguel, L. Argaud, S. Arista, L. Armand-Lefevre, MN. Bachelier, S. Balavoine, R. Baraduc, G. Barnaud, G. Beraud, D. Bertei, E. Bessede, T. Billard Pomares, C. Biron, G. Blanchard-Marche, S. Bland, J. Boileau, C. Bornstain, S. Bourdon, A. Bousquet, S. Boyer, A. Bozorg-Grayeli, L. Bret, F. Bricaire, E. Brocas, M. Brun, J. Buret, C. Burucoa, E. Cambau, G. Camuset, C. Canevet, F. Caron, A. Carricajo, I. Casin, D. Cassignard, B. Castan, C. Cazanave, T. Challan-Belval, C. Chandesris, V. Chanteperdrix-Marillier, C. Chaplain, C. Charlier-Woerther, H. Chaussade, C. Chirouze, M. Chomarat, YE Claessens, B. Clair, D. Combaux, JM Conil, H. Cordel, P. Cormier, J. Cousson, P. Cronier, E. Cua, V. Daneluzzi, A. Dao Dubremetz, A. Defarcy, N. Degand, S. Dekeyser, D. Delaune, E. Denes, D. Descamps, Jl Desmaretz, E. Devaud, MC. Di Palma, S. Diamantis, JL. Diehl, J. Dimet, A. Dinh, X. Duval, E. Descloux, J. Colot, A. Emirian, O. Epaulard, L. Escaut, C. Fabe, T. Ferry, H. Fiette, C. Flateau, N. Fonsale, E. Forestier, N. Fortineau, T. Fraisse, F. Faibis, M. Froidure, S. Gabriel-Solean, A. Gagneux-Brunon, Garandeau, M. Garcia, V. Garnier, S. Gaudry, R. Ghozzi, A. Gravet, V. Gregoire-Faucher, M. Grosset, I. Gueit, D. Guelon, C. Guillet Caruba, T. Guimard, Y. Guimard, J. Guinard, T Hadou, JP. Helene, S. Henard, B. Henry, R. Hernu, AC. Hochart, B. Hoen, N. Idri, G. Illes, X. Jacob, S. Jaffuel, D. Jan, I. Jarrin, F. Jaureguy, C. Joseph, ME. Juvin, S. Kayal, F. Lacassin, M. Lafaurie, B. Lalanne, I. Lamaury, P. Lanotte, MF. Lartigue, Y. Latorre, P. Laudat, E. Laurens, H. Laurichesse, C. Le Brun, V. Le Moing, P. Le Turnier, H. Lecuyer, S. Ledru, L. Legout, C. Legrix, A. Lemaignen, C. Lemble, L. Lemee, S. Leotard, O. Lesens, P. Lesprit, M. Levast, F. Louis, L. Quaesaet, N. Luizy, S. Males, E. Malpote, G. Martin-Blondel, V. Martinez, R. Masson, O. Matray, A. Mbadi, F. Mechai, A. Merens, MC. Meyohas, G. Michel, A. Michon, J. Mootien Yoganaden, D. Morquin, S. Mouly, N. Mrozek, S. Nguyen, Y. Nguyen, A. Odinotte, M. Ogielska, E. Oziol, B. Page, E. Parisi-Duchene, T. Pasdeloup, S. Patrat-Delon, I. Patry, A. Pechinot, I. Pelloux, S. Picot, J. Pierre, L. Piroth, C. Plassart, P. Plessis, C. Ploton, MC. Ploy, L. Portel, M. Poupard, C. Poyart, T. Prazuck, F. Raffi, A. Ramanantsoa, C. Rapp, L. Raskine, J. Raymond, M. Revest, A. Riche, S. Robaday-Voisin, F. Robin, F. Roblot, JP. Romaszko, F. Rousseau, AL. Roux, C. Royer, M. Saada, D. Salmon, C. Saroufim, JL. Schmit, M. Sebire, C. Segonds, F. Sifaoui, V. Sivadon-Tardy, N. Soismier, K. Solen, A. Sommabere, O. Son, JP. Stahl, R. Steux, S. Sunder, F. Suy, D. Tande, J. Tankovic, S. Tigaud, B. Tourrand, N. Valin, N. Van Grunderbeeck, F. Vandenesch, E. Varon, R. Vatan, C. Venot, M. Vergnaud, Vernet, M. Vidal, V. Vitrat, D. Vittecoq, F. Vuotto, A. Chabrol, J. Cabalion. Coordination and statistical analyses (Clinical trial unit, Hôpitaux Universitaires Paris Nord Val de Seine, AP-HP, Paris): GORENNE Isabelle, LAOUENAN Cédric, MARCAULT Estelle, MENTRE France, PASQUET Blandine, ROY Carine, TUBIANA Sarah F. Mentré, C. Laouenan, I. Gorenne, E. Marcault, P. Manchon, B. Pasquet, C. Roy. Scientific partnership: SPLIF, CMIT, SRLF, SFM, REIVAC, SFORL, APNET. Partners: ORP (MC PLOY), GPIP/ACTIV (Corinne Levy). Fundings: French ministry of health, Inserm, SPILF, Pfizer pharmaceutical company. Sponsor: DRCI APHP. ClinicalTrial.gov identification number: NCT01730690.
X. Duval, B. Hoen, B. Mourvillier, E. Varon, S. Tubiana, M. C. Ploy, F. Caron, Pe. Bollaert, O. Gaillot, Mk. Taha, C. Poyart, S Bonacorsi, F. Vandenesch, E. Cambau, M. Lecuit, A. Gravet, B. Frachet, T. De Broucker, D. Levy Bruhl, F. Raffi, B. Abraham, F. Ader, E. Ancel, N. Anguel, L. Argaud, S. Arista, L. Armand-Lefevre, M. N. Bachelier, S. Balavoine, R. Baraduc, G. Barnaud, G. Beraud, D. Bertei, E. Bessede, T. Billard Pomares, C. Biron, G. Blanchard-Marche, S. Bland, J. Boileau, C. Bornstain, S. Bourdon, A. Bousquet, S. Boyer, A. Bozorg-Grayeli, L. Bret, F. Bricaire, E. Brocas, M. Brun, J. Buret, C. Burucoa, E. Cambau, G. Camuset, C. Canevet, F. Caron, A. Carricajo, I. Casin, D. Cassignard, B. Castan, C. Cazanave, T. Challan-Belval, C. Chandesris, V. Chanteperdrix-Marillier, C. Chaplain, C. Charlier-Woerther, H. Chaussade, C. Chirouze, M. Chomarat, Y. E. Claessens, B. Clair, D. Combaux, J. M. Conil, H. Cordel, P. Cormier, J. Cousson, P. Cronier, E. Cua, V. Daneluzzi, A. Dao Dubremetz, A. Defarcy, N. Degand, S. Dekeyser, D. Delaune, E. Denes, D. Descamps, Jl. Desmaretz, E. Devaud, M. C. Di Palma, S. Diamantis, J. L. Diehl, J. Dimet, A. Dinh, X. Duval, E. Descloux, J. Colot, A. Emirian, O. Epaulard, L. Escaut, C. Fabe, T. Ferry, H. Fiette, C. Flateau, N. Fonsale, E. Forestier, N. Fortineau, T. Fraisse, F. Faibis, M. Froidure, S. Gabriel-Solean, A. Gagneux-Brunon, Garandeau, M. Garcia, V. Garnier, S. Gaudry, R. Ghozzi, A. Gravet, V. Gregoire-Faucher, M. Grosset, I. Gueit, D. Guelon, C. Guillet Caruba, T. Guimard, Y. Guimard, J. Guinard, T Hadou, J. P. Helene, S. Henard, B. Henry, R. Hernu, A. C. Hochart, B. Hoen, N. Idri, G. Illes, X. Jacob, S. Jaffuel, D. Jan, I. Jarrin, F. Jaureguy, C. Joseph, M. E. Juvin, S. Kayal, F. Lacassin, M. Lafaurie, B. Lalanne, I. Lamaury, P. Lanotte, M. F. Lartigue, Y. Latorre, P. Laudat, E. Laurens, H. Laurichesse, C. Le Brun, V. Le Moing, P. Le Turnier, H. Lecuyer, S. Ledru, L. Legout, C. Legrix, A. Lemaignen, C. Lemble, L. Lemee, S. Leotard, O. Lesens, P. Lesprit, M. Levast, F. Louis, L. Quaesaet, N. Luizy, S. Males, E. Malpote, G. Martin-Blondel, V. Martinez, R. Masson, O. Matray, A. Mbadi, F. Mechai, A. Merens, M. C. Meyohas, G. Michel, A. Michon, J. Mootien Yoganaden, D. Morquin, S. Mouly, N. Mrozek, S. Nguyen, Y. Nguyen, A. Odinotte, M. Ogielska, E. Oziol, B. Page, E. Parisi-Duchene, T. Pasdeloup, S. Patrat-Delon, I. Patry, A. Pechinot, I. Pelloux, S. Picot, J. Pierre, L. Piroth, C. Plassart, P. Plessis, C. Ploton, M. C. Ploy, L. Portel, M. Poupard, C. Poyart, T. Prazuck, F. Raffi, A. Ramanantsoa, C. Rapp, L. Raskine, J. Raymond, M. Revest, A. Riche, S. Robaday-Voisin, F. Robin, F. Roblot, J. P. Romaszko, F. Rousseau, A. L. Roux, C. Royer, M. Saada, D. Salmon, C. Saroufim, J. L. Schmit, M. Sebire, C. Segonds, F. Sifaoui, V. Sivadon-Tardy, N. Soismier, K. Solen, A. Sommabere, O. Son, JP. Stahl, R. Steux, S. Sunder, F. Suy, D. Tande, J. Tankovic, S. Tigaud, B. Tourrand, N. Valin, N. Van Grunderbeeck, F. Vandenesch, E. Varon, R. Vatan, C. Venot, M. Vergnaud, Vernet, M. Vidal, V. Vitrat, D. Vittecoq, F. Vuotto, A. Chabrol, J. Cabalion, S. Tubiana, F. Mentré, C. Laouénan, I. Gorenne, E. Marcault, P. Manchon, B. Pasquet, C. Roy
Funding
The cohort was funded by the French ministry of health, APHP DRCI, Inserm, French society of infectious diseases, and Pfizer pharmaceutical company.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
We have read and understood the Infection policy on declaration of interests and have no relevant interests to declare.
Ethics approval
The study was approved by the institutional Ethics Committee Ile-de-France 4 (IRB #00003835, approval #2012-16NI) and by the French Data Protection Authority (approval ECY/FLR/AR128794).
Consent to participate
All participants provided consent to participate before entering the study.
Consent for publication
Not applicable.
Additional information
The members of the COMBAT study group are mentioned in Acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akroum, S., Tubiana, S., de Broucker, T. et al. Long-term neuro-functional disability in adult patients with community-acquired bacterial meningitis. Infection 50, 1363–1372 (2022). https://doi.org/10.1007/s15010-022-01855-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-022-01855-2