Abstract
Introduction
Over the last two decades, there has been no novel acute treatment for migraine to address the large unmet medical need in Chinese patients. Lasmiditan, a novel selective serotonin 1F receptor agonist (ditan), is anticipated to bring clinical benefit in Chinese patients with migraine. The CENTURION study is a multi-country, placebo-controlled phase 3 study designed to assess the first attack efficacy and the consistency of response of lasmiditan in acute treatment of migraine. This subpopulation analysis pooled Chinese patients’ data from the primary cohort and additional extended enrollment cohort which was not published previously. This is the first analysis focusing on lasmiditan’s efficacy and safety in Chinese patients with migraine and aims to provide relevant evidence for Chinese physicians.
Methods
Patients were randomized 1:1:1 to one of the three treatment groups for four attacks: (a) lasmiditan 100 mg; (b) lasmiditan 200 mg; or (c) control group. Primary endpoints were pain freedom at 2 h (first attack) and pain freedom at 2 h in at least two out of three attacks. Secondary endpoints included pain relief, sustained pain freedom, and disability freedom.
Results
In total, 281 Chinese patients (lasmiditan 100 mg, 95; lasmiditan 200 mg, 92; control, 94) were treated for at least one migraine attack. Both doses of lasmiditan showed improvement versus placebo for pain freedom at 2 h after first attack, with lasmiditan 200 mg showing nominal significance. An early onset of effect was observed with lasmiditan versus placebo. Both doses of lasmiditan showed better results for all key secondary endpoints versus placebo. The most commonly reported treatment-emergent adverse event across all groups was dizziness.
Conclusion
In the Chinese population, lasmiditan was better than placebo for both primary endpoints and key secondary endpoints with an acceptable safety profile. No new safety signals were detected in the Chinese population. These findings are generally consistent with those observed in the CENTURION study published data and the established product profile.
Trial Registration Number
NCT03670810.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study |
Although migraine represents a huge health burden in China, over the last two decades there has been no novel acute treatment for migraine to address the large unmet medical need in Chinese patients. |
Lasmiditan, a novel selective serotonin 1F receptor agonist (ditan), is anticipated to bring great clinical benefit in Chinese patients with migraine. |
The current study is a subpopulation analysis of pooled Chinese patients’ data from the CENTURION study primary cohort and additional extended enrollment cohort which was not published previously. |
This is the first analysis focusing on lasmiditan’s efficacy and safety in Chinese patients with migraine and aims to provide relevant evidence for Chinese physicians. |
What was learned from the study |
In the Chinese population, lasmiditan was better than placebo for both primary endpoints and key secondary endpoints with an acceptable safety profile. |
The current analysis reports valuable data that demonstrates consistency with the efficacy results of the CENTURION study primary cohort in both single and multiple migraine attacks and established product profile. |
These findings could further support the use of lasmiditan in clinical practice and address the unmet medical need in the acute treatment of migraine in China. |
Introduction
Migraine is a debilitating primary headache disorder characterized by severe throbbing, pulsing headache, and generally associated with nausea and increased sensitivity to light and sound [1]. It remains the second disease among the world’s causes of disability, and the first among young women, according to Global Burden of Disease 2019 data [2]. In China, a population-based study reported that the estimated 1-year prevalence of migraine was 9.3% [3], causing 5.5 million years lived with disability (YLDs), representing a huge health burden in China [4].
For acute treatment of migraine, non-steroidal anti-inflammatory drugs (NSAIDs) are the most widely used initial medications [5], with pain freedom at 2 h occurring in 23.8–26.2% of patients [6]. On the contrary, most international guidelines recommend triptans for the acute treatment of migraine [7, 8], with pain freedom at 2 h ranging between 20% and 40% [9]. A real-world study in China showed that around 55% of patients were currently experiencing at least one adverse event (AE) with their acute medication (such as gastrointestinal effects with NSAIDs) or insufficient response to their current acute medication reported by 43% of patients [10]. Moreover, triptans are contraindicated in patients with coronary artery disease, uncontrolled hypertension, and after stroke or transient ischemic attack [11]. The aforementioned data shows that there is a substantial unmet medical need for acute treatment of migraine in China. Since no novel acute treatment for migraine was marketed in China in the last two decades, a novel acute treatment is required as an effective therapeutic option for Chinese patients with migraine.
Lasmiditan is a selective serotonin 1F (5-HT1F) receptor agonist (ditan), approved by the US Federal Drug Administration (FDA) for the acute treatment of migraine with or without aura in adults [12]. Compared to triptans, 5-HT1B/1D receptor agonists, lasmiditan has a novel mechanism of action without causing vasoconstriction [13]. In previous phase 3 studies, lasmiditan demonstrated statistically significant superiority versus placebo for pain freedom at 2 h post-dose, as well as freedom from patients’ most bothersome symptoms (MBS) [14, 15]. The phase 3 CENTURION study was conducted to assess the first attack efficacy and the consistency of response of lasmiditan as a treatment for acute migraine attacks [16]. Results from the primary cohort of the CENTURION study confirmed the early and sustained efficacy of lasmiditan 100 mg and 200 mg and demonstrated consistency of response across multiple attacks [16]. The objective of this subpopulation analysis of the CENTURION study was to demonstrate a consistent trend in the efficacy and safety of lasmiditan between the Chinese population and the primary cohort.
Methods
Patient Population and Study Design
CENTURION was a multicenter, randomized, placebo-controlled, double-blind, modified-parallel, phase 3 study. The key eligibility criteria were patients aged 18 years or older; migraine with or without aura fulfilling the IHS diagnostic criteria 1.1 or 1.2.1; a history of disabling migraine of at least 1 year; Migraine Disability Assessment Test (MIDAS) score of 11 or higher; migraine onset before the age of 50 years; and 3–8 migraine attacks per month but less than 15 headache days per month during the past 3 months. Patients with known cardiovascular risk factors or disease, with the exception of history of hemorrhagic stroke and patients on migraine preventive therapies (stable for 3 months prior to screening), were also included. The detailed inclusion and exclusion criteria were reported previously [16].
Eligible patients per stratification by country were randomized 1:1:1 to one of three treatment groups for four attacks: (a) lasmiditan 100 mg; (b) lasmiditan 200 mg; or (c) a control group, which received placebo for three attacks and lasmiditan 50 mg for either the third or fourth attack (1:1). The detailed methods and study design were previously reported [16]. To meet the registration requirements of the China regulatory agency, a maximized extended enrollment (ME2) cohort was further included in the study to assess the efficacy and safety of lasmiditan in Chinese patients. The ME2 cohort was introduced to continue enrollment if the required pre-specified number of patients was not achieved in China after the CENTURION primary cohort met its planned total sample size for the primary analysis. The current paper pooled together Chinese patients’ data from the CENTURION primary cohort and ME2 cohort to assess the efficacy, safety, and tolerability of lasmiditan in the acute treatment of migraine with or without aura.
The CENTURION study received approval from the relevant ethics committees [16] including approval from the master ethics review board: Chinese PLA general hospital. The details of the ethics review board are presented in the supplementary material. The study was conducted in accordance with the Declaration of Helsinki of 1964 and its later amendment, and all patients provided written informed consent before randomization.
Efficacy Assessments
Study Endpoints
The primary endpoints per treatment group included (1) proportion of patients who were pain free at 2 h post-dose during the first attack and (2) proportion of patients who were pain free at 2 h post-dose in at least two of three attacks (consistency of response). The key secondary endpoints were (1) the proportion of patients with pain freedom at 1 h post-dose for lasmiditan 200 mg during the first attack; (2) the proportion of patients with pain relief at 1 h and 2 h post-dose during the first attack; (3) the proportion of patients with migraine-related functional disability freedom (defined as having “not at all” recorded on a four-point scale for the question “how much is migraine interfering with normal activities?”) at 2 h post-dose during the first attack; (4) the proportion of patients with 24 h sustained pain freedom during the first attack and 48 h sustained pain freedom (lasmiditan 200 mg) during the first attack; and (5) the proportion of patients with pain relief at 2 h post-dose in at least two of three attacks (consistency of response). Other secondary endpoints also included (1) the proportion of patients who were either pain free or (2) experienced pain relief at 2 h post-dose in at least three of four attacks (consistency of response), (3) the percentage of patients who used rescue medication in the 2–24 h period during the first attack; (4) who were free of their migraine-associated MBS (MBS was chosen from three major migraine symptoms, namely nausea, photophobia, and phonophobia) at 2 h during the first attack; (5) who reported being “much better” or “very much better”, as measured using the Patient Global Impression of Change (PGIC) seven-point scale for the question “how do you feel after taking study medication?”, at 2 h and 24 h during the first attack; (6) who reported pain recurrence defined as achieving pain freedom at 2 h but having a recurrence of headache pain after 2 h, within 24 or 48 h post-dose during the first attack; and (7) the proportions of patients in the subpopulation of triptan insufficient responders (TIR) who achieved primary and secondary objectives in each group during the first attack. However, statistical analysis in the TIR Chinese subpopulation was not conducted because of the small sample size (n = 13). A pre-specified exploratory analysis was included to compare lasmiditan 50 mg and placebo using the control group data from the third and fourth attack.
Assessment of Study Endpoints
Patients were asked to record their response to study drug over a 48-h post-dose period using an electronic diary (eDiary) at prespecified time points. The method of assessments of all the study endpoints is given in detail in the CENTURION study primary cohort paper [16]. The therapeutic gain defined as the difference between the therapeutic response (pain freedom and pain relief at 2–6 h post-dose for the first attack) with lasmiditan (100 mg and 200 mg) and placebo is also presented.
Safety and Tolerability Assessments
Patients were asked at each post-dose assessment (using the eDiary), “Do you feel anything unusual since you took the study medication that you have not felt with a migraine before?” Patients who gave an affirmative response were instructed to record relevant information in a paper journal, which was to be reviewed at the subsequent visit. Treatment-emergent adverse events (TEAEs) were defined as new or worsening adverse events during the 48 h after taking a dose of the study drug. Vital signs and laboratory measures were also included in safety assessments.
Statistical Analysis
The sample size of the Chinese population was proposed to meet the local regulatory requirement with sufficient exposure in a global phase 3 study. The total sample size of the Chinese population was not intended to provide sufficient power for a region within a multiregional clinical trial; however, it was carefully pre-designed to provided sufficient chance to demonstrate a positive treatment effect in the Chinese population (i.e., showing similar efficacy trend as observed in the primary cohort).
First attack efficacy analyses were conducted using data from the intent-to-treat (ITT) Chinese population, defined as all randomized patients who used at least one dose of the study drug to treat a migraine attack of at least mild pain severity and with any post-dose pain severity assessments at or before 2 h post-dose. Consistency analyses were conducted using data from the ITT consistency Chinese population, defined as all patients who experienced at least two successes or two failures during ITT-evaluable attacks (i.e., a treated attack of at least mild pain severity with any post-dose pain severity assessments at or before 2 h post-dose). For the control group, only placebo-treated attacks were considered; for the lasmiditan groups, only the first three ITT-evaluable attacks were considered. In the primary cohort [16], logistic regression with categorical terms for treatment and geographic region were used to evaluate the proportion of patients achieving pain freedom in the first attack and to assess the consistency of response that compared lasmiditan treatment groups versus placebo. To keep the evaluation method consistent with the primary cohort, all the efficacy analyses in this Chinese subpopulation analysis were conducted using logistic regression models. For efficacy responses, patients who received rescue treatment or had missing data were defined as non-responders [16]. Given the nature of the subpopulation analysis, the outcomes (i.e., P value) of statistical inferences were intended for descriptive purpose only. In this subpopulation, post hoc P values were calculated for both the primary endpoints, and P < 0.05 was considered to indicate nominal significance.
Safety analyses were conducted using data from the safety population, which was defined as randomized patients who took at least one dose of study drug. The statistical evaluation was performed using SAS version 9.4 or higher.
Results
Patients
A total of 326 Chinese patients were randomized, including 172 patients from the ME2 cohort and 154 patients from the CENTURION primary cohort. Among them, 281 patients (lasmiditan 100 mg, 95; lasmiditan 200 mg, 92; control, 94) received at least one dose of study drug and 256 patients (lasmiditan 100 mg, 87; lasmiditan 200 mg, 87; control, 82) had first attack efficacy data. Of the 281 treated patients, 238 patients completed the study (Fig. 1).
Patient demographics and baseline characteristics were similar across the three treatment groups (lasmiditan 100 mg, lasmiditan 200 mg, and control). The mean age was 37.8 years, and the majority were female (72.2%). The mean duration of migraine history was 12.7 years, and the baseline migraine attack frequency was 4.3 per month. The mean MIDAS score was 36.4, and the proportion of patients with cardiovascular disease or risk factors was 35.9% (Table 1).
Efficacy Outcomes
Efficacy for First Attack
Patients receiving both doses of lasmiditan (100 mg and 200 mg) showed improvement compared to placebo for primary and secondary endpoints in the Chinese population (Fig. 2, supplementary Table 1).
Pain Freedom and Pain Relief at 2 h Post-dose
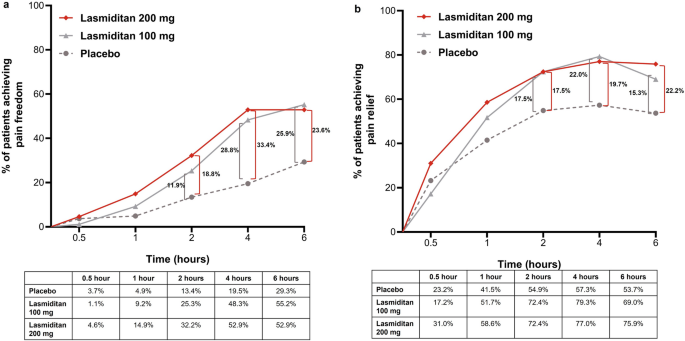
The proportion of patients receiving lasmiditan 100 mg that achieved pain freedom at 2 h post-dose after the first attack was higher (25.3%) compared to placebo (13.4%) (odds ratio, OR 2.2 [95% confidence interval, CI 1.0, 4.9]; P = 0.055), and lasmiditan 200 mg showed a nominal statistical improvement compared to placebo for pain freedom at 2 h after the first attack (32.2% versus 13.4%; OR 3.1 [95% CI 1.4, 6.7], P = 0.005) (Fig. 2). Therapeutic gains were 11.9% and 18.8% for lasmiditan 100 mg and 200 mg, respectively (Fig. 3a). Both doses of lasmiditan showed better results compared to placebo for pain relief at 2 h. The proportion of patients achieving pain relief at 2 h post-dose after the first attack were similar for lasmiditan 100 mg (72.4%) and lasmiditan 200 mg (72.4%) and higher than those for placebo (54.9%) (OR 2.2 [95% CI 1.1, 4.1]); the therapeutic gain is presented in Fig. 3b.
Early Onset of Effect
A greater proportion of patients receiving lasmiditan 100 mg achieved pain freedom at 1 h than those receiving placebo (9.2% versus 4.9%, OR 2.0 [95% CI 0.6, 6.8]; Fig. 2a) and lasmiditan 200 mg showed an earlier onset of effect with 14.9% of patients achieving pain freedom at 1 h post-dose for the first attack (OR 3.4 [95% CI 1.1, 11.0]; Fig. 2b). The proportion of patients achieving pain relief at 1 h post-dose for the first attack was higher with both doses of lasmiditan (lasmiditan 100 mg: 51.7%, OR 1.5 [95% CI 0.8, 2.8]; lasmiditan 200 mg: 58.6%, OR 2.0 [95% CI 1.1, 3.7] versus placebo, 41.5%; Fig. 2). Moreover, a higher proportion of patients who received the higher dose of lasmiditan (200 mg) achieved pain relief as early as 30 min post-dose after the first attack (lasmiditan 200 mg: 31.0%, OR 1.5 [95% CI 0.8, 3.0] versus placebo, 23.2%; Fig. 3b).
Sustained Efficacy
A sustained effect, defined as sustained pain freedom at 24 h (lasmiditan 100 mg: 14.9%, OR 1.6 [95% CI 0.6, 4.2]; lasmiditan 200 mg: 17.2%, OR 1.9 [95% CI 0.8, 4.8] versus placebo, 9.8%) and 48 h (lasmiditan 100 mg: 9.2%, OR 1.3 [95% CI 0.4, 3.9]; lasmiditan 200 mg: 18.4%, OR 2.9 [95% CI 1.1, 7.7] versus placebo, 7.3%), was seen with both doses of lasmiditan, which was better than placebo (Fig. 2). The number of patients with pain recurrence through 24 h and 48 h in each group was too low to compare the effect on recurrence among the treatment groups (24 h recurrence: placebo 2/11, lasmiditan 100 mg 4/22, lasmiditan 200 mg 5/28; 48 h recurrence: placebo 2/11, lasmiditan 100 mg 4/22, lasmiditan 200 mg 6/28).
Improvement on Associated Symptoms, Disability, and PGIC
A higher proportion of patients receiving lasmiditan (13.8% for both doses) achieved disability freedom at 2 h post-dose compared to placebo (11.0%), after the first attack. The proportions of patients who were free of their migraine-associated MBS were higher with both doses of lasmiditan (lasmiditan 100 mg: 47.1%, OR 1.5 [95% CI 0.7, 3.1]; lasmiditan 200 mg: 50.7%, OR 1.7 [95% CI 0.9, 3.5]) compared to placebo (37.5%), after the first attack (Fig. 2). A higher proportion of patients receiving lasmiditan (lasmiditan 100 mg: 41.4%, OR 2.8 [95% CI 1.4, 5.6]; lasmiditan 200 mg: 50.6%, OR 5.0 [95% CI 2.5, 10.3]) versus placebo (19.5%) reported as “feeling much/very much better” on the PGIC at 2 h post-dose, after the first attack (Fig. 2).
Consistency of Response
Both doses of lasmiditan showed better results than placebo for pain freedom and pain relief at 2 h post-dose after at least two out of three attacks and at least three out of four attacks. The proportion of patients achieving pain freedom at 2 h post-dose after at least two out of three attacks was higher with lasmiditan 100 mg (23.4%; OR 3.2 [95% CI 1.2, 8.9]; P = 0.025) and lasmiditan 200 mg (25.0%; OR 3.5 [95% CI 1.3, 9.5]; P = 0.014) than placebo (8.7%), and reached nominal significance. Compared to placebo (4.1%), higher proportions of patients who received both doses of lasmiditan achieved pain freedom at 2 h post-dose after at least three out of four attacks (lasmiditan 100 mg: 14.5%, OR 4.0 [95% CI 1.0, 15.6]; lasmiditan 200 mg: 16.1%, OR 4.6 [95% CI 1.2, 17.4]). Similarly, the proportions of patients with pain relief at 2 h post-dose after at least two out of three attacks and after at least three out of four attacks were higher with lasmiditan 100 mg (73.3%, OR 2.5 [95% CI 1.2, 5.4]; and 55.1%, OR 1.9 [95% CI 0.9, 4.1], respectively) and lasmiditan 200 mg (83.1%, OR 4.4 [95% CI 1.9, 10.1]; and 66.7%, OR 3.1 [95% CI 1.3, 7.1], respectively) compared to placebo (52.5% and 39.3%, respectively) (Fig. 2).
Lasmiditan 50 mg (Exploratory Endpoint)
For patients achieving pain freedom at 2 h post-dose, lasmiditan 50 mg (24.1%, OR 1.7 [95% CI 0.6, 5.0]) showed better results than placebo (16.4%).
Safety and Tolerability
No deaths were reported in the Chinese population including primary and ME2 cohorts. In total, 7 (2.5%) patients reported serious adverse events (SAEs) with similar incidence across treatment groups. Among them, one patient (lasmiditan 100 mg) reported a treatment-emergent SAE (migraine), which was not related to the study drug, as assessed by the investigator. TEAEs were reported in 161 (57.3%) patients, and most TEAEs were mild to moderate in severity. TEAEs related to study treatment were reported in 142 (50.5%) patients. Treatment discontinuations due to TEAEs were reported in 7 (7.4%) and 7 (7.6%) in lasmiditan 100 mg and lasmiditan 200 mg groups, respectively, and 1 (1.1%) in the placebo group (Table 2).
The most commonly reported TEAE across all groups was dizziness (lasmiditan 100 mg, 48.4%; lasmiditan 200 mg, 55.4%; placebo, 4.3%; Table 3). The incidence of TEAEs across the study (up to four attacks) is presented in Table 3. The proportions of patients who experienced at least one TEAE were higher after the first attack and decreased during subsequent attacks (Table 3).
There were no ischemic cardiovascular TEAEs reported in the Chinese population. Overall, the changes in laboratory values and vital signs were similar across the treatment groups.
Discussion
Lasmiditan is a novel high-affinity, highly selective 5-HT1F receptor agonist that acts on the trigeminal system [13]. Unlike triptans, whose actions are mediated through the activation of 5-HT1B receptors in cranial blood vessels with subsequent cranial vasoconstriction (and, therefore, are contraindicated in patients with cardiovascular disease) [17], lasmiditan showed low affinity for 5-HT1B receptors [13]. The activation of 5-HT1F receptor does not cause vasoconstriction [17, 18]. Furthermore, evidence suggests that lasmiditan can alleviate migraine through 5-HT1F agonist activity that leads to inhibition of neuropeptide and neurotransmitter release and inhibition of peripheral nervous system trigeminovascular and central nervous system pain signaling pathways [13]. Considering that triptans were approved over 20 years ago, lasmiditan could be the first novel 5-HT1F agonist for acute treatment of migraine in the last two decades.
As noted earlier, the CENTURION study primary cohort results demonstrated that lasmiditan was superior to placebo for all response endpoints during the first attack and consistency of response across multiple migraine attacks; these findings support that lasmiditan is an efficacious acute treatment for migraine [16]. The current subpopulation analysis of the CENTURION study is the first analysis to investigate lasmiditan in the Chinese population which included patients from the CENTURION primary cohort and extended Chinese patients not covered by the primary publication. The current analysis reports valuable data that demonstrates the consistency with the efficacy results published previously [16] in both single and multiple migraine attacks, with no new safety signals observed. These findings could further support the use of lasmiditan in clinical practice and resolve the unmet medical need in the acute treatment of migraine in China.
The baseline migraine characteristics of the Chinese population were generally comparable with those of the CENTURION study primary cohort. The baseline migraine attack frequency and mean MIDAS score were seemingly very close between the Chinese population and primary cohort [16] (4.3 versus 4.9 per month, 36.4 versus 31.6, respectively), representing a similar frequency of migraine attack per month and a similar degree of migraine-related disability.
In the clinical trial setting, efficacy is measured by pain freedom at 2 h before the use of a second dose of study drug or other rescue medication [19]. This endpoint is consistent with a patient’s treatment expectations, the clinician’s acute treatment goals, and independent of any confounding effect of rescue therapy [19, 20]. In the Chinese population of the CENTURION study, for first migraine attack, lasmiditan at either dose (100 mg or 200 mg) showed improvement compared to placebo for pain freedom at 2 h post-dose with consistency of response as measured by pain freedom at 2 h post-dose in at least two out of three attacks. All these primary endpoints for both doses of lasmiditan in the Chinese population, except lasmiditan 100 mg for pain freedom at 2 h during first attack, reached nominal statistical significance. These results are generally consistent with the published CENTURION study primary cohort results, pain freedom at 2 h post-dose for the first attack (lasmiditan 100 mg, 25.8%; lasmiditan 200 mg, 29.3%; versus placebo, 8.4%; P < 0.001) and in at least two out of three attacks (lasmiditan 100 mg, 14.4%; lasmiditan 200 mg, 24.4%; versus placebo, 4.3%; P < 0.001) [16].
The previous phase 3 trials of lasmiditan have reported therapeutic gain for pain freedom at 2 h post-dose of 10.1–12.9% for lasmiditan 100 mg and 16.9–17.5% for lasmiditan 200 mg [14, 15]. The CENTURION study primary cohort further demonstrated the therapeutic gain for pain freedom at 2 h post-dose was 17.4% for lasmiditan 100 mg and 20.9% for lasmiditan 200 mg [16]. Taken together, lasmiditan was reported to have generally similar therapeutic gains for pain freedom at 2 h post-dose compared to triptans (lasmiditan 100 mg, 10.1–17.4%; lasmiditan 200 mg, 16.9–20.9%; sumatriptan 50 mg, 16.9%; and sumatriptan 100 mg, 21.4%) [6, 16]. In the Chinese population of the CENTURION study, the results of therapeutic gain for pain freedom at 2 h post-dose in lasmiditan 100 mg and 200 mg groups were 11.9% and 18.8%, respectively. Meanwhile, a numerical difference of placebo effect was observed between the Chinese population and the primary cohort of the CENTURION study (pain freedom at 2 h for the first attack in placebo group: Chinese population 13.4% [95% CI 0.08–0.22] versus primary cohort 8.4% [95% CI 0.06–0.11]). However, this is just a numerical difference between both mean values with overlapping confidence interval. The impact of placebo effect on the efficacy assessment of lasmiditan was not observed.
In addition to pain freedom, a patient values rapid and early onset of pain freedom as important aspects for acute treatment of migraine [19]. In the Chinese population of the CENTURION study, the results showed an early onset for pain freedom as early as 1 h, which is consistent with the findings from three previous phase 3 trials [14,15,16]. Furthermore, the CENTURION study primary cohort showed that both doses of lasmiditan exerted a late benefit with therapeutic gain of 23–28% at 4–6 h, depending on dose and time point [16]. The results from a report that compared pain freedom at 2–8 h between lasmiditan and gepants showed similar therapeutic gains beyond 2 h across three acute treatments: lasmiditan, ubrogepant, and rimegepant [20]. These findings of therapeutic gain for late benefit between 4 and 6 h (depending on the dose and time) with lasmiditan are further evidenced by that observed in the Chinese population of the CENTURION study (Fig. 3).
Migraine is recurrent and often lifelong, which is commonly characterized by recurring attacks that can range in severity, duration, and frequency of pain. The attack frequency generally varies among different patients, which depends on the patient’s demographic and clinical characteristics [21]. Hence, the IHS guidelines recommend evaluating the consistency of response over multiple attacks in double-blind, placebo-controlled trials where response to at least four attacks is assessed, and at least one of the four attacks is treated with placebo in a randomized fashion. The guidelines further emphasize that testing the effect of an acute treatment on several migraine attacks may increase the discriminative power for efficacy when outcome measures are averaged across multiple attacks for each subject, provided that all analyzed subjects treat the same number of attacks [19]. In the Chinese population of the CENTURION study, both doses of lasmiditan showed a consistent response for pain freedom and pain relief at 2 h post-dose across at least two out of three attacks and at least three out of four attacks versus placebo. These findings of consistency of response across multiple attacks are similar to those reported in the CENTURION study primary cohort [16].
As migraine is a complex disorder with several associated symptoms, a drug effect on headache pain alone is not considered sufficient for the acute treatment of migraine. Previously, an acceptable approach to evaluate the efficacy of a drug was to demonstrate an effect on four endpoints (pain, nausea, photophobia, and phonophobia). However, per recent FDA guidance, a preferred approach aims to better align study outcomes with the symptom(s) that are of primary importance to the patient. Accordingly, a study drug should have an effect on both pain and the patient’s MBS [22]. In accordance with the FDA guidance, the phase 3 SAMURAI and SPARTAN studies evaluated MBS as a major endpoint, and both studies demonstrated statistically significant superiority of lasmiditan compared to placebo in a proportion of patients who were free of their migraine-associated MBS [14, 15]. Consistent findings for pain freedom from MBS were also reported in the CENTURION study primary cohort [16]. A similar trend was observed in the Chinese population of the CENTURION study. These findings further support the use of lasmiditan for the treatment of migraine in the Chinese population.
Taken together, a similar trend was observed across multiple endpoints and the presented OR results support the statement that a similar treatment effect to that observed in the primary cohort [16] is also demonstrated in a particular subpopulation. The results in the Chinese subpopulation showed a trend consistent with that observed in the primary cohort [16] across all primary and secondary endpoints, as demonstrated in Fig. 2 and Supplementary Table 1.
Overall, the safety results in the Chinese population were generally consistent with those of the primary cohort [16] with no new safety signals detected. The most commonly reported TEAE across all groups was dizziness. The incidence values of TEAEs reported in the Chinese population were generally consistent with those in the primary cohort. The common TEAEs in the Chinese population were similar to those in the CENTURION study primary cohort in type and severity [16]. Increased risk of cardiovascular and cerebrovascular events has been reported in patients with migraine due to the vasoconstrictive effects of triptans and ergotamines [23,24,25,26]. In line with the mechanism of action of lasmiditan [13], there were no ischemic cardio-cerebrovascular events observed with either dose of lasmiditan in the Chinese population of the CENTURION study, which indicates a favorable safety profile even in patients showing safety concerns with currently available other medications.
Patients in the control group received lasmiditan 50 mg for one attack. The results showed that lasmiditan 50 mg had positive effect compared with placebo. However, the study was not powered for or designed to fully assess the efficacy of this dose, and the 50 mg data was for exploratory purposes only as a result of the limited sample size. Hence, the results for lasmiditan 50 mg dose should be interpreted with caution.
This subpopulation analysis had a few limitations. As with the primary cohort [16], the direct comparison of TEAE findings for active treatment versus placebo across four attacks could not be evaluated because of the modified-parallel design in which lasmiditan-treated groups received lasmiditan for up to four attacks but the control group received placebo for three attacks. Secondly, on the basis of the nature of this subpopulation analysis, multiple testing control was not implemented on the tests performed. P values less than 0.05 were considered to indicate nominal significance.
Conclusions
Lasmiditan was better than placebo for both primary endpoints and key secondary endpoints with an acceptable safety profile in the Chinese population. No new safety signals were detected in the Chinese population. The findings are generally consistent with the CENTURION study primary cohort results published earlier and the established product profile.
References
Headache Classification Committee of the International Headache Society (IHS). ICHD-3. The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava, Lifting the Burden: The Global Campaign Against Headache. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137.
Yu S, Liu R, Zhao G, et al. The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache. 2012;52(4):582–91.
Yao C, Wang Y, Wang L, et al. Burden of headache disorders in China, 1990–2017: findings from the Global Burden of Disease Study 2017. J Headache Pain. 2019;20(1):102.
Steiner TJ, Jensen R, Katsarava Z, et al. Aids to management of headache disorders in primary care (2nd edition): on behalf of the European Headache Federation and Lifting the Burden: the Global Campaign against Headache. J Headache Pain. 2019;20(1):57.
Ashina M. Migraine. N Engl J Med. 2020;383(19):1866–76.
Evers S, Afra J, Frese A, et al. EFNS guideline on the drug treatment of migraine—revised report of an EFNS task force. Eur J Neurol. 2019;16(9):968–81.
Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3–20.
Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia. 2002;22(8):633–58 (Erratum in: Cephalalgia. 2003;23(1):71).
Zhao H, Zhang L, Ford J, et al. PND64 treatment patterns and real-world outcomes among patients with episodic migraine in China. ISPOR. 2012;24(Supplement 1):S171.
Diener HC. The risks or lack thereof of migraine treatments in vascular disease. Headache. 2020;60(3):649–53.
Lamb YN. Lasmiditan: first approval. Drugs. 2019;79(18):1989–96.
Clemow DB, Johnson KW, Hochstetler HM, Ossipov MH, Hake AM, Blumenfeld AM. Lasmiditan mechanism of action - review of a selective 5-HT1F agonist. J Headache Pain. 2020;21(1):71.
Kuca B, Silberstein SD, Wietecha L, et al. Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology. 2018;91:e2222–32.
Goadsby PJ, Wietecha LA, Dennehy EB, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142(7):1894–904.
Ashina M, Reuter U, Smith T, et al. Randomized, controlled trial of lasmiditan over four migraine attacks: findings from the CENTURION study. Cephalalgia. 2021;41(3):294–304.
Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, MaassenVanDenBrink A. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88–97.
Vila-Pueyo M. Targeted 5-HT1F therapies for migraine. Neurotherapeutics. 2018;15(2):291–303.
Diener HC, Tassorelli C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults: fourth edition. Cephalalgia. 2019;39(6):687–710.
Doty EG, Krege JH, Pohl G, Case M, Dowsett SA, Tepper SJ. Pain freedom at 2 to 8 hours with lasmiditan: a comparison with rimegepant and ubrogepant. Headache. 2020;60(8):1793–6.
Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–91.
US Food and Drug Administration. Migraine: developing drugs for acute treatment guidance for industry. https://www.fda.gov/files/drugs/published/Migraine--Developing-Drugs-for-Acute-Treatment.pdf. Accessed 28 July 2021.
Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357: j2099.
Mahmoud AN, Mentias A, Elgendy AY, et al. Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open. 2018;8(3): e020498.
Humphrey P, Feniuk W, Perren MJ, et al. Serotonin and migraine. Ann N Y Acad Sci. 1990;600:587–98.
Mitsikostas D, Tfelt-Hansen P. Targeting to 5-HT1F receptor subtype for migraine treatment: lessons from the past, implications for the future. Cent Nerv Syst Agents Med Chem. 2012;12(4):241–9.
Acknowledgements
The authors thank Bin Zhang, MS, for data analysis support for Fig. 2; Yan Cheng, MD, Hui Liu, MD, and Shiying Zhong, MD, from Eli Lilly and Company for their valuable review of this article. The authors thank the participants of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The CENTURION study was funded by Eli Lilly and Company. This analysis in subpopulation of Chinese patients and the Rapid Service Fee for publication was funded and supported by Eli Lilly and Company.
Medical Writing, Editorial, and Other Assistance
The authors thank Nan Yao, PhD, from Eli Lilly and Company for project management, medical writing, and editorial assistance. The authors thank Deepika Kajarekar, from Syneos Health, for medical writing support and editorial assistance. The medical writing support was funded by Eli Lilly and Company.
Author Contributions
Shengyuan Yu, Quan Hu, and Tingmin Yu were involved in study design. Shengyuan Yu, Tingmin Yu, Li He, Xiaosu Yang, Jiying Zhou, Guogang Luo, Hebo Wang, Hongru Zhao, and Quan Hu were involved in collection and interpretation of data. Shengyuan Yu, Quan Hu, Fei Ji, and Li He drafted and reviewed the manuscript. Fei Ji performed the statistical analyses. All authors read and approved the final manuscript.
List of Investigators
The list of investigators is provided in supplementary material.
Prior Presentation
Part of the results in this manuscript were presented as an abstract and ePoster in the International Headache Congress 2021, 8–12 September 2021 in Berlin (Germany).
Disclosures
Quan Hu and Fei Ji are full-time employees at Eli Lily and Company. Shengyuan Yu serves as associated editor of the Journal of Headache and Pain and as Member of the International Headache Society. Tingmin Yu, Li He, Xiaosu Yang, Jiying Zhou, Guogang Luo, Hebo Wang, Hongru Zhao and Shengyuan Yu report financial relationships with Eli Lilly and Company for clinical research fee.
Compliance with Ethics Guidelines
The CENTURION study received approval from the relevant ethics committees including approval from the master ethics review board: Chinese PLA general hospital. The details of the ethics review board are presented in the supplementary material. The study was conducted in accordance with the Declaration of Helsinki of 1964 and its later amendment, and all patients provided written informed consent before randomization.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yu, T., He, L., Yang, X. et al. Efficacy and Safety of Lasmiditan as a Novel Acute Treatment in Chinese Patients with Migraine: A Subpopulation Analysis of the Randomized Controlled Phase 3 CENTURION Trial. Neurol Ther 11, 1269–1283 (2022). https://doi.org/10.1007/s40120-022-00369-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00369-1