Abstract
Introduction
Amongst populations of northern European ancestry, HFE-associated haemochromatosis is a common genetic disorder characterised by iron overload. In the absence of treatment, excess iron is stored in parenchymal tissues, causing morbidity and mortality. Population screening programmes may increase early diagnosis and reduce associated disease. No contemporary health economic evaluation has been published for Australia. The objective of this study was to identify cost-effective screening strategies for haemochromatosis in the Australian setting.
Methods
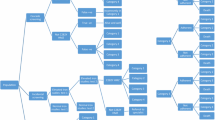
A Markov model using probabilistic decision analysis was developed comparing four adult screening strategies: the status quo (cascade and incidental screening), genotyping with blood and buccal samples and transferrin saturation followed by genotyping (TfS). Target populations were males (30 years) and females (45 years) of northern European ancestry. Cost-effectiveness was estimated from the government perspective over a lifetime horizon.
Results
All strategies for males were cost-effective compared to the status quo. The incremental costs (standard deviation) associated with genotyping (blood) were AUD7 (56), TfS AUD15 (45) and genotyping (buccal) AUD63 (56), producing ICERs of AUD1673, 4103 and 15,233/quality-adjusted life-year (QALY) gained, respectively. For females, only the TfS strategy was cost-effective, producing an ICER of AUD10,195/QALY gained. Approximately 3% of C282Y homozygotes were estimated to be identified with the status quo approach, compared with 40% with the proposed screening strategies.
Conclusion
This model estimated that genotyping and TfS strategies are likely to be more cost-effective screening strategies than the status quo.


Similar content being viewed by others
References
Worwood M. Inborn errors of metabolism: iron. Br Med Bull. 1999;55(3):556–67.
Allen K. Hereditary haemochromatosis—diagnosis and management. Aust Fam Physician. 2010;39(12):938–41.
Wood MJ, Skoien R, Powell LW. The global burden of iron overload. Hepatol Int. 2009;3(3):434–44.
Gagne G, et al. Hereditary hemochromatosis screening: effect of mutation penetrance and prevalence on cost-effectiveness of testing algorithms. Clin Genet. 2007;71(1):46–58.
Adams PC, Valberg LS. Screening blood donors for hereditary hemochromatosis: decision analysis model comparing genotyping to phenotyping. Am J Gastroenterol. 1999;94(6):1593–600.
Allen KJ, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358(3):221–30.
Whitlock EP, et al. Screening for hereditary hemochromatosis: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2006;145(3):209–23.
Asberg A, et al. Screening for hemochromatosis: high prevalence and low morbidity in an unselected population of 65,238 persons. Scand J Gastroenterol. 2001;36(10):1108–15.
Thorstensen K, et al. Screening for C282Y homozygosity in a Norwegian population (HUNT2): the sensitivity and specificity of transferrin saturation. Scand J Clin Lab Invest. 2010;70(2):92–7.
Handa P, Kowdley KV. Glyceronephosphate O-acyltransferase as a hemochromatosis modifier gene: another iron in the fire? Hepatology. 2015;62(2):337–9.
McLaren CE, et al. Exome sequencing in HFE C282Y homozygous men with extreme phenotypes identifies a GNPAT variant associated with severe iron overload. Hepatology. 2015;62(2):429–39.
Barton J, Edwards CQ. Hemochromatosis: genetics, patholphysiology, diagnosis and treatment. Cambridge: Cambridge University Press; 2000.
Byrnes V, et al. The underdiagnosis of hereditary haemochromatosis: lack of presentation or penetration, expectations based on a study of relatives of symptomatic probands. Gastroenterology. 2000;118(4):A997.
Mundy L, Merlin T. Population genetic screening for haemochromatosis: identifying asymptomatic “at risk” homozygous individuals. In: AHTA, editor. Horizon scanning prioritising summary, vol 1. A.H.T.A. Adelaide: AGDHA; 2004.
Adams P, Brissot P, Powell LW. EASL international consensus conference on haemochromatosis. J Hepatol. 2000;33(3):485–504.
Allen KJ. Population genetic screening for hereditary haemochromatosis: are we a step closer? Med J Aust. 2008;189(6):300–1.
Delatycki MB, et al. Use of community genetic screening to prevent HFE-associated hereditary haemochromatosis. Lancet. 2005;366(9482):314–6.
Adams P, et al. Screening for iron overload: lessons from the hemochromatosis and iron overload screening (HEIRS) study. Can J Gastroenterol. 2009;23(11):769–72.
Gasser B, et al. Hereditary hemochromatosis: presenting manifestations and diagnostic delay. Rev Med Interne. 2013;35(3):160–5. doi:10.1016/j.revmed.2013.02.041.
Caro JJ, et al. Modeling good research practices—overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Med Decis Making. 2012;32(5):667–77.
de Graaff B, et al. A Systematic review and narrative synthesis of health economic studies conducted for hereditary haemochromatosis. Appl Health Econ Policy. 2015. doi:10.1007/s40258-015-0189-y.
Adams PC, Speechley M. The effect of arthritis on the quality of life in hereditary hemochromatosis. J Rheumatol. 1996;23(4):707–10.
de Graaff B, et al. Quality of life utility values for hereditary haemochromatosis in Australia. Health Qual Life Outcomes. 2016;14(31). doi:10.1186/s12955-016-0431-9.
Asberg A, et al. Benefit of population-based screening for phenotypic hemochromatosis in young men. Scand J Gastroenterol. 2002;37(10):1212–9.
Adams P, et al. Screening blood donors for hereditary hemochromatosis: decision analysis model based on a 30-year database. Gastroenterol Clin North Am. 1995;108:177–88.
Adams P, Kertesz A, Valberg L. Screening for hemochromatosis in children of homozygotes: prevalence and cost-effectiveness. Hepatology. 1995;22(6):1720–7.
de Graaff B, Si L, Neil AL, et al. Population screening for hereditary haemochromatosis in Australia: construction and validation of a state-transition cost-effectiveness model. Pharmacoecon Open. 2016. doi:10.1007/s41669-016-0005-0.
Adams P, Brissot P, Powell L. EASL international consensus conference on haemochromatosis—part II. Expert document. J Hepatol. 2000;33(3):487–96.
Allen KJ, et al. Healthiron: a longitudinal population study defining the burden of disease in HFE-associated hereditary hemochromatosis. Am J Hematol. 2007;82(6):537.
Gurrin LC, et al. The natural history of serum iron indices for HFE C282Y homozygosity associated with hereditary hemochromatosis. Gastroenterology. 2008;135(6):1945–52.
Australian Bureau of Statistics. Life table, states, territories and Australia, 2011–2013. 2014 5th July 2015; Available from: http://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/3302.0.55.001Main+Features12011-2013?OpenDocument.
Crooks CJ, et al. The epidemiology of haemochromatosis: a population-based study. Aliment Pharmacol Ther. 2009;29(2):183–92.
Elmberg M, et al. Increased mortality risk in patients with phenotypic hereditary hemochromatosis but not in their first-degree relatives. Gastroenterology. 2009;137(4):1301–9.
Rogowski WH. The cost-effectiveness of screening for hereditary hemochromatosis in Germany: a remodeling study. Med Decis Making. 2009;29(2):224–38.
Phatak PD, Guzman G, Woll JE, Robeson A, Phelps CE. Cost-effectiveness of screening for hereditary hemochromatosis. Arch Intern Med. 1994;154(7):769–76.
Adams PC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352(17):1769–78.
Merryweather-Clarke AT, et al. A rapid non-invasive method for the detection of the haemochromatosis C282Y mutation. Br J Haematol. 1997;99(2):460–3.
Bradley LA, et al. Hereditary haemochromatosis mutation frequencies in the general population. J Med Screen. 1998;5(1):34–6.
Baty D, et al. Development of a multiplex ARMS test for mutations in the HFE gene associated with hereditary haemochromatosis. J Clin Pathol. 1998;51(1):73–4.
Soloway RD, et al. Inhibitors present in blood do not inhibit PCR from buccal cell preparations: case report. Vivo. 1999;13(6):453–4.
Nisselle AE, et al. Implementation of HaemScreen, a workplace-based genetic screening program for hemochromatosis. Clin Genet. 2004;65(5):358–67.
Australian Institute of Health and Welfare and Australia. Department of Health and Ageing. National Bowel Cancer Screening Program monitoring report, Phase 2, July 2008–June 2011. Cancer series. Canberra: Australian Institute of Health and Welfare; 2012. xiii.
Australian Institute for Health and Welfare, Cervical screening in Australia 2012–2013, in Cancer series. Canberra: Australian Institute for Health and Welfare; 2015.
Hicken BL, Tucker DC, Barton JC. Patient compliance with phlebotomy therapy for iron overload associated with hemochromatosis. Am J Gastroenterol. 2003;98(9):2072–7.
de Graaff B, et al. Costs associated with hereditary haemochromatosis in Australia: a cost of illness study. Aust Health Rev. 2016. doi:10.1071/AH15188.
Australian Government Department of Health. Medicare benefits schedule book. Canberra: Australian Government Department of Health; 2014.
Australian Government Department of Health, schedule of pharmaceutical benefits 1 January 2015–31 January 2015. Canberra: Australian Government Department of Health; 2015.
Independent Hospital Pricing Authority. National hospital cost data collection Australian public hospitals cost report 2011–2012, round 16. Independent Hospital Pricing Authority; 2014.
Australian Government Department of Health and Ageing. Technical guidelines for preparing assessment reports for the Medical Services Advisory Committee—service type: therapeutic (Version 1.2), A.G.D.o.H.a. In: Ageing, editor. Canberra: Australian Government Department of Health and Ageing; 2013.
Eddy DM, et al. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force-7. Value Health. 2012;15(6):843–50.
Briggs AH, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working group-6. Med Decis Making. 2012;32(5):722–32.
Henry DA, Hill SR, Harris A. Drug prices and value for money: the Australian pharmaceutical benefits scheme. Jama. 2005;294(20):2630–2.
Scott N, Iser DM, Thompson AJ, Doyle JS, Hellard ME. Cost-effectiveness of treating chronic hepatitis C virus with direct-acting antivirals in people who inject drugs in Australia. J Gastroenterol Hepatol. 2016;31(4):872–82.
Bardou-Jacquet E, et al. Decreased cardiovascular and extrahepatic cancer-related mortality in treated patients with mild HFE hemochromatosis. J Hepatol. 2015;62(3):682–9.
Smith DP, et al. Prostate cancer and prostate-specific antigen testing in New South Wales. Med J Aust. 2008;189(6):315–8.
Nisselle AE, Delatycki MB, Collins V, Metcalfe S, Aitken MA, du Sart D, et al. Implementation of HaemScreen, a workplace-based genetic screening program for hemochromatosis. Clin Genet. 2004;65(5):358–67.
Gurrin L. 2015, personal communication.
Bryant J, Cooper K, Picot J, Clegg A, Roderick P, Rosenberg W, et al. A systematic review of the clinical validity and clinical utility of DNA testing for hereditary haemochromatosis type 1 in at-risk populations. J Med Genet. 2008;45(8):513–8.
Acknowledgements
The authors would like to acknowledge the support provided by the Centre for Health Economics Research and Evaluation at the University of Technology Sydney. In particular, we would like to thank Professor Jane Hall, Associate Professor Stephen Goodall and Dr. Rob Anderson (University of Exeter Medical School).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No financial support was provided for this project nor for the preparation of the manuscript. Barbara de Graaff was supported by a Ph.D. Australian Postgraduate Research Award scholarship provided by the Australian Government: this body did not have any role in the study.
Support
Support was provided by members of the Centre for Health Economics Research and Evaluation at the University of Technology Sydney [Professor Jane Hall, Associate Professor Stephen Goodall and Dr. Rob Anderson (University of Exeter)] by providing preliminary work on an economic model for haemochromatosis. None of the CHERE work is included in this manuscript.
Conflict of interest
Barbara de Graaff, Amanda Neil, Kristy Sanderson, Lei Si, Kwang Chien Yee, Lyle Gurrin and Andrew J. Palmer have no conflicts of interest to declare that are directly relevant to the content of this review.
Author contributions
Barbara de Graaff planned and constructed the model, conducted the model simulations and sensitivity analyses, and prepared the manuscript. Lei Si assisted with construction and validation of the model and assisted with preparation of the manuscript. Amanda Neil and Kristy Sanderson contributed to constructing the model, interpreting results and assisted with preparation of the manuscript. Kwang Chien Yee assisted with assessing the model for face validity and assisted with preparation of the manuscript. Lyle Gurrin assisted with the construction and validity of the model, and assisted with preparation of the manuscript. Andrew Palmer assisted with planning and constructing the model, conducting simulations and sensitivity analyses, and assisted with preparation of the manuscript.
Rights and permissions
About this article
Cite this article
de Graaff, B., Neil, A., Si, L. et al. Cost-Effectiveness of Different Population Screening Strategies for Hereditary Haemochromatosis in Australia. Appl Health Econ Health Policy 15, 521–534 (2017). https://doi.org/10.1007/s40258-016-0297-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-016-0297-3