Abstract
Background
Decisions made by the National Institute for Health and Care Excellence (NICE) exert an influence on the allocation of resources within ‘fixed’ National Health Service budgets. Yet guidance for different types of health interventions is handled via different ‘programmes’ within NICE, which follow different methods and processes.
Objective
The objective of this research was to identify differences in the processes and methods of NICE health technology assessment programmes and to explore how these could impact on allocative efficiency within the National Health Service.
Methods
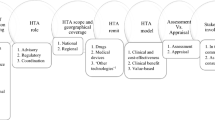
Data were extracted from the NICE technology appraisal programme, medical technologies guidance, diagnostic assessment programme, highly specialised technologies programme, and clinical guidelines process and methods manuals to undertake a systematic comparison. Five qualitative interviews were carried out with NICE members of staff and committee members to explore the reasons for the differences found.
Results
The main differences identified were in the required evidence review period, or lack thereof, mandatory funding status, the provision of a reference case for economic evaluation, the requirement for and the type of economic analysis undertaken, and the decision making criteria used for appraisal.
Conclusion
Many of the differences found can be justified on grounds of practicality and relevance to the health technologies under assessment. Nevertheless, from a strict utilitarian view, there are several potential areas of inefficiency that could lead to the misallocation of resources within the National Health Service, although some of these might be eliminated or reduced if an egalitarian view is taken. The challenge is determining where society is willing to trade health gains between different people.
Similar content being viewed by others
References
Knapp M. The economics of social care. Hampshire: Macmillan Publishers Ltd; 1984.
National Institute for Health and Care Excellence. Social value judgements: principles for the development of NICE guidance. 2nd ed. London: National Institute for Health and Care Excellence; 2008.
Dolan P. Utilitarianism and the measurement and aggregation of quality-adjusted life years. Health Care Anal. 2001;9:65–76.
McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26(9):733–44.
Claxton K, Martin S, Soares M, et al. Methods for the estimation of the NICE cost effectiveness threshold. CHE Research Paper 81: revised report following referees’ comments. York: Centre for Health Economics, University of York; 2013.
Barnsley P, Towse A, Schaffer SK, Sussex J. Critique of CHE Research Paper 81: methods for the estimation of the NICE cost effectiveness threshold. London: Office of Health Economics; 2013: Occasional Paper 13/01.
Chapman A, Taylor C, Girling A. Are the UK systems of innovation and evaluation of medical devices compatible? The role of NICE’s medical technologies evaluation programme (MTEP). Appl Health Econ Health Policy. 2014;12(4):347–57.
Green W, Hutton J. Health technology assessments in England: an analysis of the NICE medical technologies evaluation programme. Eur J Health Econ. 2014;15:449–52.
National Institute for Health and Care Excellence. Guide to the processes of technology appraisal: process and methods guide. London: National Institute for Health and Care Excellence; 2013.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013: process and methods guides. London: National Institute for Health and Care Excellence; 2013.
National Institute for Health and Care Excellence. Medical technologies evaluation programme: process guide. London: National Institute for Health and Care Excellence; 2011.
National Institute for Health and Care Excellence. Medical technologies evaluation programme: methods guide. London: National Institute for Health and Care Excellence; 2011.
National Institute for Health and Care Excellence. Diagnostics assessment programme manual. London: National Institute for Health and Care Excellence; 2011.
National Institute for Health and Care Excellence. Interim addendum to the diagnostics assessment programme manual: access proposals from the sponsors of diagnostic technologies. London: National Institute for Health and Care Excellence; 2011.
National Institute for Health and Care Excellence. Interim addendum to replace existing section 9: guidance reviews, in DAP programme manual. London: National Institute for Health and Care Excellence; 2011.
National Institute for Health and Care Excellence. Interim process and methods of the highly specialised technologies programme. London: National Institute for Health and Care Excellence; 2013.
National Institute for Health and Care Excellence. Developing NICE guidelines: the manual. Process and methods guide. London: National Institute for Health and Care Excellence; 2015.
National Institute for Health and Care Excellence. Developing NICE guidelines: the manual appendices A to I. Process and methods guide. London: National Institute for Health and Care Excellence; 2015.
National Institute for Health and Care Excellence. Developing NICE guidelines: the manual appendix H. Process and methods guide. London: National Institute for Health and Care Excellence; 2015.
National Institute for Health and Care Excellence. Interventional procedures programme: process guide. London: National Institute for Health and Care Excellence; 2009.
National Institute for Health and Care Excellence. Interventional procedures programme: methods guide. London: National Institute for Health and Clinical Excellence; 2009.
Regulation (EU) No. 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC. Official Journal of the European Union; 2014.
Drummond M, Griffin A, Tarricone R. Economic evaluation for devices and drugs: same or different? Value Health. 2009;12(4):402–6.
Kiristis A, Redekop W. The economic evaluation of medical devices: challenges ahead. Appl Health Econ Health Policy. 2013;11(1):15–26.
Sorenson C, Tarricone R, Siebert M, Drummond M. Applying health economics for policy decision making: do devices differ from drugs? Europace. 2011;13(Suppl. 2):ii54–8.
Taylor R, Iglesias C. Assessing the clinical and cost-effectiveness of medical devices and drugs: same or different? Value Health. 2009;12(4):402–4.
Drummond M, Wilson D, Kanavos P, et al. Assessing the economic challenges posed by orphan drugs. Int J Technol Assess. 2007;23(1):36–42.
Hughes D, Tunnage B, Yeo S. Drugs for exceptionally rare diseases: do they deserve special status for funding? QJM. 2005;98:829–36.
Desser AS, Gyrd-Hansen D, Olsen JA, et al. Societal views on orphan drugs: cross sectional survey of Norwegians aged 40 to 67. BMJ. 2010;341:c4715.
Linley WG, Hughes DA. Societal views on NICE, cancer drugs fund and value-based pricing criteria for prioritising medicines: a cross sectional survey of 4118 adults in Great Britain. Health Econ. 2013;22:948–64.
National Institute for Health and Clinical Excellence. NICE citizens council report: ultra orphan drugs. London: National Institute for Health and Clinical Excellence; 2004.
Tordrup D, Tzouma V, Kanavos P. Orphan drug considerations in health technology assessment in eight European countries. Int J Public Health. 2014;1(3):83–97.
Trueman P, Drummond M, Hutton J. Developing guidance for budget impact analysis. Pharmacoeconomics. 2001;19(6):609–21.
Cookson R, McDaid D, Maynard A. Wrong sign, NICE mess: is national guidance distorting allocation of resources? BMJ. 2001;323:743–5.
Birch S, Gafni A. The ‘NICE’ approach to technology assessment: an economics perspective. Health Care Manag Sci. 2004;7:35–41.
Appleby J, Devlin N, Parkin D. NICE’s cost effectiveness threshold: how high should it be? BMJ. 2007;335:358–9.
Acknowledgements
This project was undertaken by the first author while on placement at the Office of Health Economics, as part of the requirements of the MSc International Health Policy (Health Economics), LSE dissertation. All authors are grateful to colleagues at NICE for their support and input into this work. Views expressed in this article are those of the authors, and do not necessarily reflect those of NICE.
Author contributions
EC conducted the analysis and drafted the initial manuscript while on placement at Office of Health Economics, as part of the requirements of the MSc International Health Policy (Health Economics), LSE dissertation. GM and AC proposed to undertake the research and supervised EC. All authors contributed to revisions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The work was funded by the Office of Health Economics from the annual research grant it receives from the Association of the British Pharmaceutical Industry (ABPI).
Conflict of interest
The ABPI did not have any input into this work. Amanda Cole and Nancy Devlin declare no conflicts of interest. Emma Cowles conducted initial research whilst on a placement at the Office of Health Economics and currently works at the National Guideline Centre, Royal College of Physicians. The National Guideline Centre had no input into this work. Grace Marsden is a member of the NICE Guidelines Standing Committee C. The comparison of HTA programmes presented here was undertaken before this appointment commenced, and the views should not be considered to be those held by NICE or the updates committee.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cowles, E., Marsden, G., Cole, A. et al. A Review of NICE Methods and Processes Across Health Technology Assessment Programmes: Why the Differences and What is the Impact?. Appl Health Econ Health Policy 15, 469–477 (2017). https://doi.org/10.1007/s40258-017-0309-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-017-0309-y