Abstract
Background
Health state utility values (HSUVs) identified from utility elicitation studies are widely used in pharmacoeconomic evaluations for chronic hepatitis C (CHC) and are particularly instrumental in health technology assessment (HTA) evaluations such as those from the National Institute for Health and Care Excellence (NICE).
Objective
The aim of this study was to identify HSUVs used in cost-utility analyses (CUAs) for CHC in Europe and to evaluate the impact of HSUV selection on cost-effectiveness results in terms of the incremental cost per quality-adjusted life-year (QALY) gained (ICER).
Methods
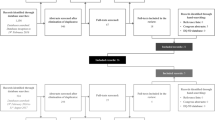
A systematic search of pharmacoeconomic evaluations for CHC was updated in the MEDLINE and EMBASE databases for the periods 2012–2017 and 2017–2020. Data on health states, HSUVs, and utility elicitation studies were extracted. The difference in HSUVs of the same health state in different CUAs, and the difference between HSUVs of one health state and of the interlink health state in the same CUAs, were calculated. A quality assessment was performed to evaluate the selection of HSUVs in CUAs. Sets of HSUVs identified were used in a reconstructed CUA model to assess the impact on the ICER.
Results
Twenty-six CUAs conducted in European countries and referring to 17 utility elicitation studies were included. The difference in HSUVs of the same health state in different CUAs ranged from 0.021 (liver transplant) to 0.468 (decompensated cirrhosis). The difference between HSUVs of one health state and of the interlink health state of the next disease severity level was calculated between the health states of F0–F1/mild and F2–F3/moderate (n = 11, 0.040–0.110), F2–F3/moderate and F4/compensated cirrhosis (n = 18, 0.027–0.130), compensated cirrhosis and decompensated cirrhosis (n = 22, 0.020–0.100), decompensated cirrhosis and hepatocellular carcinoma (n = 24, 0.000–0.200), hepatocellular carcinoma and liver transplant in the first year (n = 17, − 0.329 to 0.170) and liver transplant in the first and subsequent years (n = 17, − 0.340 to 0.000). The utility elicitation study selected by most CUAs (n = 11) was recommended as the source of HSUVs, at least for the CUAs conducted in the UK, based on the results of quality assessment. Seven sets of HSUVs were generated to fit the reconstructed model and changed the results of the incremental analysis from being cost effective to not being cost effective (ICER ranging from £2460 to £24,954 per QALY gained), and to being dominated in the UK setting.
Conclusions
The CUAs for CHC were found to apply to various HSUVs from different utility elicitation studies in the same health state. This variability in HSUVs has the potential to significantly affect ICER and ICER-based reimbursement decisions. A rigorous selection of HSUVs in CUAs to inform healthcare resource allocation is suggested for future studies of CUAs and for guideline development.


Similar content being viewed by others
Data Availability Statement
All data relating to this systematic review are included in the article and/or its supplementary material. The model used in the study can be made available to researchers upon reasonable request.
References
Han R, Zhou J. PIN11—comparison of clinical burden of HCV infection between Asia and Europe: an overview of systematic review, in ISPOR Asia Pacific 2018. Tokyo: Elsevier; 2018.
World Health Organization. Global Hepatitis report, 2017 [cited 30 Aug 2018]. http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. Accessed 15 May 2018.
Han R, et al. Prevalence of hepatitis C infection among the general population and high-risk groups in the EU/EEA: a systematic review update. BMC Infect Dis. 2019;19(1):655.
Martin NK, et al. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Curr Opin HIV AIDS. 2015;10(5):374–80.
Han R, Zhou J. PIN19 - a cost comparison of treating chronic hepatitis c patients with sofosbuvir-based regimens. Value Health. 2018;21:S224.
de Graaff B, et al. Uptake of and expenditure on direct-acting antiviral agents for hepatitis C treatment in Australia. Appl Health Econ Health Policy. 2018;16(4):495–502.
McDonald SA, et al. Projections of the healthcare costs and disease burden due to hepatitis C infection under different treatment policies in Malaysia, 2018-2040. Appl Health Econ Health Policy. 2018;16(6):847–57.
Cipriano LE, Goldhaber-Fiebert JD. Population Health and Cost-Effectiveness Implications of a “Treat All” Recommendation for HCV: A Review of the Model-Based Evidence. MDM Policy Pract. 2018;3(1):2381468318776634.
Townsend R, et al. Structural frameworks and key model parameters in cost-effectiveness analyses for current and future treatments of chronic hepatitis C. Value Health. 2011;14(8):1068–77.
Chhatwal J, He T, Lopez-Olivo MA. Systematic review of modelling approaches for the cost effectiveness of hepatitis c treatment with direct-acting antivirals. Pharmacoeconomics. 2016;34(6):551–67.
Luhnen M, et al. Health economic evaluations of sofosbuvir for treatment of chronic hepatitis C: a systematic review. Appl Health Econ Health Policy. 2016;14(5):527–43.
Castro R, et al. Cost-effectiveness of diagnostic and therapeutic interventions for chronic hepatitis C: a systematic review of model-based analyses. BMC Med Res Methodol. 2018;18(1):53.
Puig-Junoy J, et al. Cost-utility analysis of second-generation direct-acting antivirals for hepatitis C: a systematic review. Expert Rev Gastroenterol Hepatol. 2018;12(12):1251–63.
Szilberhorn L, Kalo Z, Agh T. Cost-effectiveness of second-generation direct-acting antiviral agents in chronic HCV infection: a systematic literature review. Antivir Ther. 2019;24(4):247–59.
Vellopoulou A, et al. Cost utility of telaprevir-PR (peginterferon-ribavirin) versus boceprevir-PR and versus PR alone in chronic hepatitis C in The Netherlands. Appl Health Econ Health Policy. 2014;12(6):647–59.
Leidner AJ, et al. Assessing the effect of potential reductions in non-hepatic mortality on the estimated cost-effectiveness of hepatitis C treatment in early stages of liver disease. Appl Health Econ Health Policy. 2017;15(1):65–74.
Elbasha EH, et al. Cost-effectiveness analysis of boceprevir for the treatment of chronic hepatitis C virus genotype 1 infection in Portugal. Appl Health Econ Health Policy. 2013;11(1):65–78.
Ruggeri M, et al. Cost-effectiveness analysis of early treatment of chronic HCV with sofosbuvir/velpatasvir in Italy. Appl Health Econ Health Policy. 2018;16(5):711–22.
Graham RF, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27(1):209–12.
Cardoso H, Silva M. Health-related quality of life in chronic hepatitis C. GE Port J Gastroenterol. 2017;24(2):55–7.
Cardoso H, et al. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J Hepatol. 2016;65(5):1070–1.
Innes HA, et al. Toward a more complete understanding of the association between a hepatitis C sustained viral response and cause-specific outcomes. Hepatology. 2015;62(2):355–64.
Zeuzem S, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343(23):1666–72.
Queneau PE, et al. Treatment of mild chronic hepatitis C with interferon alpha-2b: results of a multi-centre randomized study in 80 patients. Eur J Gastroenterol Hepatol. 2001;13(2):143–7.
Verbaan H, et al. High sustained response rate in patients with histologically mild (low grade and stage) chronic hepatitis C infection. A randomized, double blind, placebo controlled trial of interferon alpha-2b with and without ribavirin. Eur J Gastroenterol Hepatol. 2002;14:627–33.
Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296(13):716–21.
Mathes T, et al. Methods of international health technology assessment agencies for economic evaluations: a comparative analysis. BMC Health Serv Res. 2013;13:371.
National Institute for Health and Care Excellence (NICE). 7. Assessing cost effectiveness. The guidelines manual PMG6. 2012. Available from: https://www.nice.org.uk/process/pmg6/chapter/assessing-cost-effectiveness. Accessed 2 July 2020.
Haute Autorite de Sante (HAS). Choices in methods for economic evaluation. France: HAS; 2012. Available at: https://www.has-sante.fr/portail/upload/docs/application/pdf/2012-10/choices_in_methods_for_economic_evaluation.pdf. Accessed 4 Apr 2019.
Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada. 4th: CADTH; 2017. Available at: https://www.cadth.ca/about-cadth/how-we-do-it/methods-and-guidelines/guidelines-for-the-economic-evaluation-of-health-technologies-canada. Accessed 2 July 2020.
Fukuda T. Analysis guidelines for cost-effectiveness evaluation at the central social insurance medical council. 2016. Available at: https://www.researchgate.net/publication/309369832_Development_of_an_Official_Guideline_for_the_Economic_Evaluation_of_DrugsMedical_Devices_in_Japan.
China Guidelines for Pharmacoeconomic Evaluation Working Group. China Guidelines for Pharmacoeconomic Evaluation; 2019.
Bundesausschuss, G. Verfahrensordnung des Gemeinsamen Bundesausschusses 2020 [cited 17 Jul 2020]. https://www.g-ba.de/informationen/richtlinien/42/. Accessed 1 May 2020.
Oliva-Moreno J, et al. Economic evaluation for pricing and reimbursement of new drugs in spain: fable or desideratum? Value Health. 2019;23(1):25–31.
House, Ways and Means Committee. H.R.3590–Patient Protection and Affordable Care Act. 2010.
Institute for Clinical and Economic Review. Cost-Effectiveness, the QALY, and the evLYG. 2020. https://icer-review.org/methodology/qaly/. Accessed 19 Sep 2019.
Sadasivan S. Health technology assessment in the Asia Pacific region. Int J Technol Assess Health Care. 2009;25(Suppl 1):196–201.
Oortwijn W, Mathijssen J, Banta D. The role of health technology assessment on pharmaceutical reimbursement in selected middle-income countries. Health Policy. 2010;95(2–3):174–84.
Tantivess S, et al. Health Technology Assessment capacity development in low- and middle-income countries: experiences from the international units of HITAP and NICE. F1000Research. 2017;6:2119.
World Health Organization. 2015 Global Survey on Health Technology Assessment by National Authorities. Geneva: World Health Organization; 2015.
Xie F, et al. Toward a centralized, systematic approach to the identification, appraisal, and use of health state utility values for reimbursement decision making: introducing the Health Utility Book (HUB). Med Decis Making. 2019;39(4):370–8.
Zhou J, et al. Systematic review of utility values used in the pharmacoeconomic evaluations for schizophrenia: implications on cost-effectiveness results. J Mark Access Health Policy. 2019;7(1):1648973.
Centers for Reviews and Dissemination. CRD’s guidance for undertaking reviews in health care. York: Centers for Reviews and Dissemination; 2009.
Shepherd J, Waugh N, Hewitson P. Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review. Health Technol Assess. 2000;4(33):1–67.
Shepherd J, et al. Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2004;8(39):iii-iv (1-125).
Shepherd J, et al. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2007;11(11):1–205 (iii).
Annemans L, et al. A health economic model to assess the long term effects and cost-effectiveness of PEG IFN alpha-2a in hepatitis C virus infected patients. Acta Gastroenterol Belg. 2004;67(1):1–8.
Garcia-Contreras F, et al. Cost-effectiveness of chronic hepatitis C treatment with thymosin alpha-1. Arch Med Res. 2006;37(5):663–73.
Hornberger J, et al. The economics of treating chronic hepatitis C patients with peginterferon alpha-2a (40 kDa) plus ribavirin presenting with persistently normal aminotransferase. J Viral Hepat. 2006;13(6):377–86.
Lidgren M, et al. Productivity improvements in hepatitis C treatment: impact on efficacy, cost, cost-effectiveness and quality of life. Scand J Gastroenterol. 2007;42(7):867–77.
Lin WA, Tarn YH, Tang SL. Cost-utility analysis of different peg-interferon alpha-2b plus ribavirin treatment strategies as initial therapy for naive Chinese patients with chronic hepatitis C. Aliment Pharmacol Ther. 2006;24(10):1483–93.
Nakamura J, et al. The cost-effectiveness of the new protocol reflecting rapid virologic response to peginterferon alpha-2b and ribavirin for chronic hepatitis C. Eur J Gastroenterol Hepatol. 2007;19(9):733–9.
Salomon JA, et al. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290(2):228–37.
Sullivan SD, et al. Cost-effectiveness of combination peginterferon alpha-2a and ribavirin compared with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Am J Gastroenterol. 2004;99(8):1490–6.
Sullivan SD, et al. Cost effectiveness of peginterferon alpha-2a plus ribavirin versus interferon alpha-2b plus ribavirin as initial therapy for treatment-naive chronic hepatitis C. Pharmacoeconomics. 2004;22(4):257–65.
Wong J, Nevens F. Cost-effectiveness of peginterferon alfa-2b plus ribavirin compared to interferon alfa-2b plus ribavirin as initial treatment of chronic hepatitis C in Belgium. Acta Gastroenterol Belg. 2002;65(2):110–1.
McEwan P, Kim R, Yuan Y. Assessing the cost utility of response-guided therapy in patients with chronic hepatitis C genotype 1 in the UK using the MONARCH model. Appl Health Econ Health Policy. 2013;11(1):53–63.
Nerich V, et al. Critical appraisal of health-state utility values used in breast cancer-related cost-utility analyses. Breast Cancer Res Treat. 2017;164(3):527–36.
Johnson SJ, et al. Economic evaluation of ombitasvir/paritaprevir/ritonavir and dasabuvir for the treatment of chronic genotype 1 hepatitis c virus infection. J Med Econ. 2016;19(10):983–94.
Macken L, et al. Efficacy of direct-acting antivirals: UK real-world data from a well-characterised predominantly cirrhotic HCV cohort. J Med Virol. 2019;91(11):1979–88.
Buxton MJ, et al. Modelling in economic evaluation: an unavoidable fact of life. Health Econ. 1997;6(3):217–27.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. London: National Institute for Health and Care Excellence; 2013.
Bennett H, et al. Hepatitis C disease transmission and treatment uptake: impact on the cost-effectiveness of new direct-acting antiviral therapies. Eur J Health Econ. 2017;18(8):1001–11.
Martin NK, et al. Prioritization of HCV treatment in the direct-acting antiviral era: An economic evaluation. J Hepatol. 2016;65(1):17–25.
McEwan P, et al. A clinician’s guide to the cost and health benefits of hepatitis C cure assessed from the individual patient perspective. Eur J Gastroenterol Hepatol. 2017;29(2):208–14.
McEwan P, et al. Estimating the cost-effectiveness of daclatasvir + sofosbuvir versus sofosbuvir + ribavirin for patients with genotype 3 hepatitis C virus. Cost Eff Resour Alloc. 2017;15:15.
van Santen DK, et al. Cost-effectiveness of hepatitis C treatment for people who inject drugs and the impact of the type of epidemic; extrapolating from Amsterdam, The Netherlands. PLoS One. 2016;11(10):e0163488.
Wisloff T, et al. Economic evaluation of direct-acting antivirals for hepatitis C in Norway. Pharmacoeconomics. 2018;36(5):591–601.
Sbarigia U, et al. Economic study of the value of expanding HCV treatment capacity in Germany. BMJ Open Gastroenterol. 2017;4(1):e000130.
Stahmeyer JT, et al. Cost-effectiveness of treating hepatitis C with sofosbuvir/ledipasvir in Germany. PLoS One. 2017;12(1):e0169401.
Deuffic-Burban S, et al. Cost-effectiveness and budget impact of interferon-free direct-acting antiviral-based regimens for hepatitis C treatment: the French case. J Viral Hepat. 2016;23(10):767–79.
Maunoury F, et al. Cost-effectiveness analysis of elbasvir-grazoprevir regimen for treating hepatitis C virus genotype 1 infection in stage 4-5 chronic kidney disease patients in France. PLoS One. 2018;13(3):e0194329.
Ruggeri M, et al. Economic evaluation of the hepatitis C virus treatment extension to early-stage fibrosis patients: evidence from the PITER real-world cohort. Value Health. 2018;21(7):783–91.
Rolli FR, et al. Economic evaluation of Zepatier for the management of HCV in the Italian scenario. Eur J Health Econ. 2018;19(9):1365–74.
Kondili LA, et al. Modeling cost-effectiveness and health gains of a “universal” versus “prioritized” hepatitis C virus treatment policy in a real-life cohort. Hepatology. 2017;66(6):1814–25.
Turnes J, Dominguez-Hernandez R, Casado MA. Value and innovation of direct-acting antivirals: long-term health outcomes of the strategic plan for the management of hepatitis C in Spain. Rev Esp Enferm Dig. 2017;109(12):809–17.
Gimeno-Ballester V, et al. Cost-effectiveness analysis of therapeutic options for chronic hepatitis C genotype 3 infected patients. Expert Rev Gastroenterol Hepatol. 2017;11(1):85–93.
Ruggeri M, et al. Cost-effectiveness analysis of Daclatasvir/Sofosbuvir for the treatment of the HCV patients failed after the first line with second generation of DAAs in Italy. Expert Rev Pharmacoecon Outcomes Res. 2019;19(3):363–74.
Heath K. Cost-effectiveness analysis of treatment timing considering the future entry of lower-cost generics for hepatitis C. Clinicoecon Outcomes Res. 2018;10:539–50.
Popping S, et al. Early treatment of acute hepatitis C infection is cost-effective in HIV-infected men-who-have-sex-with-men. PLoS One. 2019;14(1):e0210179.
Restelli U, et al. Cost-effectiveness analysis of the use of daclatasvir + sofosbuvir + ribavirin (16 weeks and 12 weeks) vs sofosbuvir + ribavirin (16 weeks and 24 weeks) for the treatment of cirrhotic patients affected with hepatitis C virus genotype 3 in Italy. Eur J Health Econ. 2018;19(1):37–44.
Buti M, et al. Cost-effectiveness analysis of ledipasvir/sofosbuvir in patients with chronic hepatitis C: treatment of patients with absence or mild fibrosis compared to patients with advanced fibrosis. J Viral Hepat. 2017;24(9):750–8.
Cortesi PA, et al. Management of treatment-naive chronic hepatitis C genotype 1 patients: a cost-effectiveness analysis of treatment options. J Viral Hepat. 2015;22(2):175–83.
Cure S, et al. Cost-effectiveness of sofosbuvir plus ribavirin with or without pegylated interferon for the treatment of chronic hepatitis C in Italy. J Med Econ. 2015;18(9):678–90.
Cure S, Guerra I, Dusheiko G. Cost-effectiveness of sofosbuvir for the treatment of chronic hepatitis C-infected patients. J Viral Hepat. 2015;22(11):882–9.
McEwan P, et al. The cost-effectiveness of daclatasvir-based regimens for the treatment of hepatitis C virus genotypes 1 and 4 in the UK. Eur J Gastroenterol Hepatol. 2016;28(2):173–80.
Wright M, et al. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess. 2006;10(21):1–113 (iii).
Kind P, Hardman G, Macran S. UK population norms for EQ5D. York: The University of York Centre for Health Economics; 1999.
Hzode C, et al. Daclatasvir in combination with peginterferon Alfa-2a and ribavirin for treatment-naive patients with HCV genotype 4 infection: phase 3 COMMAND-4 results. Open Forum Infectious Diseases. 2014;1(Suppl 1):S233.
Sulkowski MS, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–21.
McDonald SA, et al. Decrease in health-related quality of life associated with awareness of hepatitis C virus infection among people who inject drugs in Scotland. J Hepatol. 2013;58(3):460–6.
McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making. 2008;28(4):582–92.
Siebert U, et al. Cost effectiveness of peginterferon alpha-2b plus ribavirin versus interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52(3):425–32.
Chong CA, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98(3):630–8.
Schwarzinger M, et al. P0745: EQ-5D utility index in French patients with chronic hepatits C (CHC) infection: severe comorbidities and perceived progression of CHC infection matter more than actual liver disease stage. J Hepatol. 2015;62(Suppl 2):S605.
Tengs TO, Lin TH. A meta-analysis of utility estimates for HIV/AIDS. Med Decis Making. 2002;22(6):475–81.
McGreal-bellone A, et al. PHS60 health-related quality of life in HIV/HCV co-infected patients in Ireland. Value Health. 2012;15(7):A528–9.
Cortesi PA, et al. The impact of type of liver conditions on the patients’ health related quality of life. Value in Health. 2013;16(7):A500.
Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–20.
Pol S, et al. P0747: health related quality of life and utility values in chronic hepatitis C patients: a cross-sectional study in France, the UK and Germany. J Hepatol. 2015;62:S606.
Buchanan-Hughes AM, et al. Health state utility values measured using the EuroQol 5-dimensions questionnaire in adults with chronic hepatitis C: a systematic literature review and meta-analysis. Qual Life Res. 2019;28(2):297–319.
Papaioannou D, Brazier J, Paisley S. NICE DSU Technical Support Document 9: The identification, review and synthesis of health state utility values from the literature. 2010.
Wright M, et al. Measurement and determinants of the natural history of liver fibrosis in hepatitis C virus infection: a cross sectional and longitudinal study. Gut. 2003;52(4):574–9.
Bowling A, et al. Short Form 36 (SF-36) Health Survey questionnaire: which normative data should be used? Comparisons between the norms provided by the Omnibus Survey in Britain, the Health Survey for England and the Oxford Healthy Life Survey. J Public Health Med. 1999;21(3):255–70.
Fischer JA, et al. Quality of life of people who inject drugs: characteristics and comparisons with other population samples. Qual Life Res. 2013;22(8):2113–21.
Chahal HS, et al. Cost-effectiveness of early treatment of hepatitis C virus genotype 1 by stage of liver fibrosis in a US treatment-naive population. JAMA Intern Med. 2016;176(1):65–73.
Chhatwal J, et al. Cost-effectiveness and budget impact of hepatitis C Virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397–406.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Ru Han contributed to the literature search, acquisition of data, analysis and interpretation of the data, and the first draft of the manuscript. Clément François and Junwen Zhou contributed to the critical revision of the manuscript for important intellectual content. Clément François and Mondher Toumi contributed to the supervision of the study. Marcus Bashford and Malgorzata Biernikiewicz contributed to the writing or technical editing of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Funding
The authors received no specific funding for this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, R., François, C. & Toumi, M. Systematic Review of Health State Utility Values Used in European Pharmacoeconomic Evaluations for Chronic Hepatitis C: Impact on Cost-Effectiveness Results. Appl Health Econ Health Policy 19, 29–44 (2021). https://doi.org/10.1007/s40258-020-00600-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-020-00600-w