Abstract
Background and Objective
The rate of depression in patients with HIV is higher than in the general population. The use of antidepressants can have a beneficial effect, improving antiretroviral therapy adherence and consequently their efficacy and safety. Efavirenz and protease inhibitor boosted with ritonavir are major components of the antiretroviral therapy and are inducers and/or inhibitors of several cytochrome P450 (CYP) isoforms. Although antidepressants are prescribed to a significant proportion of patients treated with antiretrovirals, there are limited clinical data on drug-drug interactions. The aim of this study was to predict the magnitude of drug-drug interactions among efavirenz, boosted protease inhibitors and the most commonly prescribed antidepressants using an in vitro-in vivo extrapolation (IVIVE) model simulating virtual clinical trials.
Methods
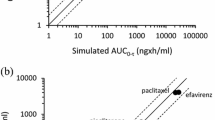
In vitro data describing the chemical characteristics, and absorption, distribution, metabolism and elimination (ADME) properties of efavirenz, boosted protease inhibitors and the most commonly prescribed antidepressants were obtained from published literature or generated by standard methods. Pharmacokinetics and drug-drug interaction were simulated using the full physiologically based pharmacokinetic model implemented in the Simcyp™ ADME simulator. The robustness of our modeling approach was assessed by comparing the magnitude of simulated drug-drug interactions using probe drugs to that observed in clinical studies.
Results
Simulated pharmacokinetics and drug-drug interactions were in concordance with available clinical data. Although the simulated drug-drug interactions with antidepressants were overall weak to moderate according to the classification of the US FDA, fluoxetine and venlafaxine represent better candidates from a pharmacokinetic standpoint for patients on efavirenz and venlafaxine or citalopram for patients on boosted protease inhibitors.
Conclusion
The modest magnitude of interaction could be explained by the fact that antidepressants are substrates of multiple isoforms and thus metabolism can still occur through CYPs that are weakly impacted by efavirenz or boosted protease inhibitors. These findings indicate that IVIVE is a useful tool for predicting drug-drug interactions and designing prospective clinical trials, giving insight into the variability of exposure, sample size and time-dependent induction or inhibition.

Similar content being viewed by others
References
Eller LS, Bunch EH, Wantland DJ, Portillo CJ, Reynolds NR, Nokes KM, et al. Prevalence, correlates, and self-management of HIV-related depressive symptoms. AIDS Care. 2010;22(9):1159–70.
Starace F, Ammassari A, Trotta MP, Murri R, De Longis P, Izzo C, et al. Depression is a risk factor for suboptimal adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S136–9.
Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114(7):573–80.
Tsai AC, Weiser SD, Petersen ML, Ragland K, Kushel MB, Bangsberg DR. A marginal structural model to estimate the causal effect of antidepressant medication treatment on viral suppression among homeless and marginally housed persons with HIV. Arch Gen Psychiatry. 2010;67(12):1282–90.
Bauer M, Monz BU, Montejo AL, Quail D, Dantchev N, Demyttenaere K, et al. Prescribing patterns of antidepressants in Europe: results from the Factors Influencing Depression Endpoints Research (FINDER) study. Eur Psychiatry. 2008;23(1):66–73.
Marzolini C, Elzi L, Gibbons S, Weber R, Fux C, Furrer H, et al. Prevalence of comedications and effect of potential drug–drug interactions in the Swiss HIV Cohort Study. Antivir Ther. 2010;15(3):413–23.
Stedman CA. Current prospects for interferon-free treatment of hepatitis C in 2012. J Gastroenterol Hepatol. 2013;28(1):38–45.
Darwich AS, Neuhoff S, Jamei M, Rostami-Hodjegan A. Interplay of metabolism and transport in determining oral drug absorption and gut wall metabolism: a simulation assessment using the “Advanced Dissolution, Absorption, Metabolism (ADAM)” model. Curr Drug Metab. 2010;11(9):716–29.
Sun D, Lennernas H, Welage LS, Barnett JL, Landowski CP, Foster D, et al. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm Res. 2002;19(10):1400–16.
Poulin P, Theil FP. Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J Pharm Sci. 2002;91(1):129–56.
Ogburn ET, Jones DR, Masters AR, Xu C, Guo Y, Desta Z. Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab Dispos. 2010;38(7):1218–29.
Belanger AS, Caron P, Harvey M, Zimmerman PA, Mehlotra RK, Guillemette C. Glucuronidation of the antiretroviral drug efavirenz by UGT2B7 and an in vitro investigation of drug–drug interaction with zidovudine. Drug Metab Dispos. 2009;37(9):1793–6.
Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306(1):287–300.
Rittweger M, Arasteh K. Clinical pharmacokinetics of darunavir. Clin Pharmacokinet. 2007;46(9):739–56.
Koudriakova T, Iatsimirskaia E, Utkin I, Gangl E, Vouros P, Storozhuk E, et al. Metabolism of the human immunodeficiency virus protease inhibitors indinavir and ritonavir by human intestinal microsomes and expressed cytochrome P4503A4/3A5: mechanism-based inactivation of cytochrome P4503A by ritonavir. Drug Metab Dispos. 1998;26(6):552–61.
Wempe MF, Anderson PL. Atazanavir metabolism according to CYP3A5 status: an in vitro–in vivo assessment. Drug Metab Dispos. 2011;39(3):522–7.
von Moltke LL, Greenblatt DJ, Grassi JM, Granda BW, Venkatakrishnan K, Duan SX, et al. Citalopram and desmethylcitalopram in vitro: human cytochromes mediating transformation, and cytochrome inhibitory effects. Biol Psychiatry. 1999;46(6):839–49.
Ring BJ, Eckstein JA, Gillespie JS, Binkley SN, VandenBranden M, Wrighton SA. Identification of the human cytochromes p450 responsible for in vitro formation of R- and S-norfluoxetine. J Pharmacol Exp Ther. 2001;297(3):1044–50.
Obach RS, Cox LM, Tremaine LM. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study. Drug Metab Dispos. 2005;33(2):262–70.
Stormer E, von Moltke LL, Shader RI, Greenblatt DJ. Metabolism of the antidepressant mirtazapine in vitro: contribution of cytochromes P-450 1A2, 2D6, and 3A4. Drug Metab Dispos. 2000;28(10):1168–75.
Fogelman SM, Schmider J, Venkatakrishnan K, von Moltke LL, Harmatz JS, Shader RI, et al. O- and N-demethylation of venlafaxine in vitro by human liver microsomes and by microsomes from cDNA-transfected cells: effect of metabolic inhibitors and SSRI antidepressants. Neuropsychopharmacology. 1999;20(5):480–90.
Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A. The Simcyp population-based ADME simulator. Expert Opin Drug Metab Toxicol. 2009;5(2):211–23.
Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, et al. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320(1):72–80.
Hariparsad N, Nallani SC, Sane RS, Buckley DJ, Buckley AR, Desai PB. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J Clin Pharmacol. 2004;44(11):1273–81.
Rekic D, Roshammar D, Mukonzo J, Ashton M. In silico prediction of efavirenz and rifampicin drug–drug interaction considering weight and CYP2B6 phenotype. Br J Clin Pharmacol. 2011;71(4):536–43.
von Moltke LL, Greenblatt DJ, Granda BW, Giancarlo GM, Duan SX, Daily JP, et al. Inhibition of human cytochrome P450 isoforms by nonnucleoside reverse transcriptase inhibitors. J Clin Pharmacol. 2001;41(1):85–91.
FDA Advisory Committee. Atazanavir BMS-232632. Briefing Document. http://www.fda.gov/ohrms/dockets/ac/03/briefing/3950B1_01_BristolMyersSquibb-Atazanavir.pdf. Accessed 7 Mar 2013
Hyland R, Dickins M, Collins C, Jones H, Jones B. Maraviroc: in vitro assessment of drug–drug interaction potential. Br J Clin Pharmacol. 2008;66(4):498–507.
Foti RS, Rock DA, Wienkers LC, Wahlstrom JL. Selection of alternative CYP3A4 probe substrates for clinical drug interaction studies using in vitro data and in vivo simulation. Drug Metab Dispos. 2010;38(6):981–7.
Kenworthy KE, Bloomer JC, Clarke SE, Houston JB. CYP3A4 drug interactions: correlation of 10 in vitro probe substrates. Br J Clin Pharmacol. 1999;48(5):716–27.
Turpeinen M, Raunio H, Pelkonen O. The functional role of CYP2B6 in human drug metabolism: substrates and inhibitors in vitro, in vivo and in silico. Curr Drug Metab. 2006;7(7):705–14.
Eagling VA, Tjia JF, Back DJ. Differential selectivity of cytochrome P450 inhibitors against probe substrates in human and rat liver microsomes. Br J Clin Pharmacol. 1998;45(2):107–14.
Jackson A, Hill A, Puls R, Else L, Amin J, Back D, et al. Pharmacokinetics of plasma lopinavir/ritonavir following the administration of 400/100 mg, 200/150 mg and 200/50 mg twice daily in HIV-negative volunteers. J Antimicrob Chemother. 2011;66(3):635–40.
Di Giambenedetto S, De Luca A, Villani P, Bacarelli A, Ragazzoni E, Regazzi M, et al. Atazanavir and lopinavir with ritonavir alone or in combination: analysis of pharmacokinetic interaction and predictors of drug exposure. HIV Med. 2008;9(4):239–45.
Boffito M, Miralles D, Hill A. Pharmacokinetics, efficacy, and safety of darunavir/ritonavir 800/100 mg once-daily in treatment-naive and -experienced patients. HIV Clin Trials. 2008;9(6):418–27.
Vrouenraets SM, Wit FW, van Tongeren J, Lange JM. Efavirenz: a review. Expert Opin Pharmacother. 2007;8(6):851–71.
Kirby BJ, Collier AC, Kharasch ED, Whittington D, Thummel KE, Unadkat JD. Complex drug interactions of HIV protease inhibitors 1: inactivation, induction, and inhibition of cytochrome P450 3A by ritonavir or nelfinavir. Drug Metab Dispos. 2011;39(6):1070–8.
Muirhead GJ, Wulff MB, Fielding A, Kleinermans D, Buss N. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Br J Clin Pharmacol. 2000;50(2):99–107.
Abel S, Russell D, Taylor-Worth RJ, Ridgway CE, Muirhead GJ. Effects of CYP3A4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers. Br J Clin Pharmacol. 2008;65(Suppl 1):27–37.
Aarnoutse RE, Kleinnijenhuis J, Koopmans PP, Touw DJ, Wieling J, Hekster YA, et al. Effect of low-dose ritonavir (100 mg twice daily) on the activity of cytochrome P450 2D6 in healthy volunteers. Clin Pharmacol Ther. 2005;78(6):664–74.
Park J, Vousden M, Brittain C, McConn DJ, Iavarone L, Ascher J, et al. Dose-related reduction in bupropion plasma concentrations by ritonavir. J Clin Pharmacol. 2010;50(10):1180–7.
Kirby BJ, Collier AC, Kharasch ED, Dixit V, Desai P, Whittington D, et al. Complex drug interactions of HIV protease inhibitors 2: in vivo induction and in vitro to in vivo correlation of induction of cytochrome P450 1A2, 2B6, and 2C9 by ritonavir or nelfinavir. Drug Metab Dispos. 2011;39(12):2329–37.
Abel S, Jenkins TM, Whitlock LA, Ridgway CE, Muirhead GJ. Effects of CYP3A4 inducers with and without CYP3A4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers. Br J Clin Pharmacol. 2008;65(Suppl 1):38–46.
Robertson SM, Maldarelli F, Natarajan V, Formentini E, Alfaro RM, Penzak SR. Efavirenz induces CYP2B6-mediated hydroxylation of bupropion in healthy subjects. J Acquir Immune Defic Syndr. 2008;49(5):513–9.
Food and Drug Administration. Guidance for industry. Drug interaction studies—study design, data analysis, implications for dosing, and labeling recommendations. 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf. Accessed 7 Mar 2013
Fuller RW, Snoddy HD, Krushinski JH, Robertson DW. Comparison of norfluoxetine enantiomers as serotonin uptake inhibitors in vivo. Neuropharmacology. 1992;31(10):997–1000.
Weiss J, Herzog M, Konig S, Storch CH, Ketabi-Kiyanvash N, Haefeli WE. Induction of multiple drug transporters by efavirenz. J Pharmacol Sci. 2009;109(2):242–50.
Dixit V, Hariparsad N, Li F, Desai P, Thummel KE, Unadkat JD. Cytochrome P450 enzymes and transporters induced by anti-human immunodeficiency virus protease inhibitors in human hepatocytes: implications for predicting clinical drug interactions. Drug Metab Dispos. 2007;35(10):1853–9.
Uhr M, Grauer MT. abcb1ab P-glycoprotein is involved in the uptake of citalopram and trimipramine into the brain of mice. J Psychiatr Res. 2003;37(3):179–85.
Wang JS, Zhu HJ, Gibson BB, Markowitz JS, Donovan JL, DeVane CL. Sertraline and its metabolite desmethylsertraline, but not bupropion or its three major metabolites, have high affinity for P-glycoprotein. Biol Pharm Bull. 2008;31(2):231–4.
Mahar Doan KM, Humphreys JE, Webster LO, Wring SA, Shampine LJ, Serabjit-Singh CJ, et al. Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. J Pharmacol Exp Ther. 2002;303(3):1029–37.
Bachmeier CJ, Beaulieu-Abdelahad D, Ganey NJ, Mullan MJ, Levin GM. Induction of drug efflux protein expression by venlafaxine but not desvenlafaxine. Biopharm Drug Dispos. 2011;32(4):233–44.
Siccardi M, Almond L, Schipani A, Csajka C, Marzolini C, Wyen C, et al. Pharmacokinetic and pharmacodynamic analysis of efavirenz dose reduction using an in vitro–in vivo extrapolation model. Clin Pharmacol Ther. 2012;92(4):494–502.
Kis O, Zastre JA, Hoque MT, Walmsley SL, Bendayan R. Role of drug efflux and uptake transporters in atazanavir intestinal permeability and drug–drug interactions. Pharm Res. 2012 [Epub ahead of print].
Holmstock N, Mols R, Annaert P, Augustijns P. In situ intestinal perfusion in knockout mice demonstrates inhibition of intestinal p-glycoprotein by ritonavir causing increased darunavir absorption. Drug Metab Dispos. 2010;38(9):1407–10.
Fahmi OA, Maurer TS, Kish M, Cardenas E, Boldt S, Nettleton D. A combined model for predicting CYP3A4 clinical net drug–drug interaction based on CYP3A4 inhibition, inactivation, and induction determined in vitro. Drug Metab Dispos. 2008;36(8):1698–708.
Kumar GN, Rodrigues AD, Buko AM, Denissen JF. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277(1):423–31.
Oganesian A, Shilling AD, Young-Sciame R, Tran J, Watanyar A, Azam F, et al. Desvenlafaxine and venlafaxine exert minimal in vitro inhibition of human cytochrome P450 and P-glycoprotein activities. Psychopharmacol Bull. 2009;42(2):47–63.
Feng B, Mills JB, Davidson RE, Mireles RJ, Janiszewski JS, Troutman MD, et al. In vitro P-glycoprotein assays to predict the in vivo interactions of P-glycoprotein with drugs in the central nervous system. Drug Metab Dispos. 2008;36(2):268–75.
Summerfield SG, Read K, Begley DJ, Obradovic T, Hidalgo IJ, Coggon S, et al. Central nervous system drug disposition: the relationship between in situ brain permeability and brain free fraction. J Pharmacol Exp Ther. 2007;322(1):205–13.
Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug–drug interactions: an update. Curr Drug Metab. 2002;3(1):13–37.
Gutierrez MM, Rosenberg J, Abramowitz W. An evaluation of the potential for pharmacokinetic interaction between escitalopram and the cytochrome P450 3A4 inhibitor ritonavir. Clin Ther. 2003;25(4):1200–10.
Janssen-Cilag Ltd. Prezista Summary of Product Characteristics. 2012.
Bristol-Myers Squibb Company. Sustiva Prescribing Information. 2010.
Acknowledgments
Andrew Owen has received honoraria for lectures or advisory board meetings and/or research support research funding by Abbott Laboratories, AstraZeneca, GlaxoSmithKline, Merck, Pfizer and Roche Pharmaceuticals. David Back and Saye Khoo have received honoraria for lectures or advisory board meetings and/or research support and research funding by Merck, Gilead, BMS, Tibotec and ViiV. Marco Siccardi has received financial support from Simcyp Ltd. Lisa Almond is an employee of Simcyp Ltd. Catia Marzolini, Kay Seden and Anna Kirov declared no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siccardi, M., Marzolini, C., Seden, K. et al. Prediction of drug-drug Interactions Between Various Antidepressants and Efavirenz or Boosted Protease Inhibitors Using a Physiologically Based Pharmacokinetic Modelling Approach. Clin Pharmacokinet 52, 583–592 (2013). https://doi.org/10.1007/s40262-013-0056-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-013-0056-7