Abstract
Background and Objectives
This study implemented pharmacokinetic/pharmacodynamic modelling to support the clinical development of RBP-6000, a new, long-acting, sustained-release formulation of buprenorphine for the treatment of opioid dependence. Such a formulation could offer advantages over existing buprenorphine pharmacotherapy by improving patient compliance and reducing the diversion of the product.
Methods
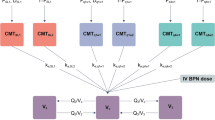
A population pharmacokinetic model was developed using 36 opioid-dependent subjects who received single subcutaneous doses of RBP-6000. Another pharmacokinetic/pharmacodynamic model was developed using μ-opioid receptor occupancy (µORO) data to predict efficacy of RBP-6000 after repeated doses. It was also assessed how buprenorphine plasma concentrations were correlated with opioid withdrawal symptoms and hydromorphone agonist blockade data from 15 heroin-dependent subjects.
Results
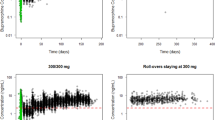
The resulting pharmacokinetic model accurately described buprenorphine and norbuprenorphine plasma concentrations. A saturable maximum effect (E max) model with 0.67 ng/mL effective concentration at 50 % of maximum (EC50) and 91 % E max best described µORO versus buprenorphine plasma concentrations. Linear relationships were found among µORO, withdrawal symptoms and blockade of agonist effects.
Conclusion
Previously published findings have demonstrated µORO ≥70 % is needed to achieve withdrawal suppression and blockade of opioid agonist subjective effects. Model simulations indicated that a 200 mg RBP-6000 dose should achieve 2–3 ng/mL buprenorphine average concentrations and desired efficacy.





Similar content being viewed by others
References
Katz NP, et al. Prescription opioid abuse: challenges and opportunities for payers. Am J Manag Care. 2013;19:295–302.
Katz NP, et al. Foundations of opioid risk management. Clin J Pain. 2007;23:103–18.
Paulozzi LJ, Ryan GW. Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31:506–11.
Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011;59:1–51.
Substance Abuse and Mental Health Services Administration, Drug Abuse Warning Network (2011). National estimates of drug-related emergency department visits. HHS publication no. (SMA) 13-4760, DAWN series D-39. Rockville: Substance Abuse and Mental Health Services Administration; 2013.
Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharm Ther. 1994;55:569–80.
SUBOXONE® sublingual film label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022410s015lbl.pdf. Accessed 31 Jan 2014.
Weiss RD. Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction. 2004;99:1382–92.
Boothby LA, Doering PL. Buprenorphine for the treatment of opioid dependence. Am J Health-Syst Pharm. 2007;64:266–72.
R Foundation for Statistical Computing (2009). R: a language and environment for statistical computing. http://www.R-project.org. Accessed 14 Dec 2013.
Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM user’s guides, 1989–2013. Ellicott City: Icon Development Solutions; 2013.
Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Meth Prog Biomed. 1999;58:51–64.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Meth Prog Biomed. 2005;79:241–57.
Food and Drug Administration (1999). Guidance for industry: population pharmacokinetics. http://www.fda.gov/downloads/Drugs/Guidances/UCM072137.pdf. Accessed 16 Dec 2013.
Hooker AC, Staatz CE, Karlsson MO. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res. 2007;24(12):2187–97.
Kobayashi K, et al. Human buprenorphine N-dealkylation is catalyzed by cytochrome P450 3A4. Drug Metab Disp. 1998;26:818–21.
Parke J, Holford NH, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Meth Prog Biomed. 1999;59:19–29.
Greenwald MK, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28:2000–9.
Greenwald MK, et al. Buprenorphine duration of action: mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry. 2007;61:101–10.
Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34:711–26.
Greenwald MK, Steinmiller CL. Imaging opioid receptors: applications to substance use disorders. In: Dean R, Negus SS, Bilsky E, editors. Opioid receptors and antagonists: from bench to clinic. New York: Humana Press 2009; p. 45–65.
Gomeni R, Heidbreder C, Fudala PJ, Nasser AF. A model-based approach to characterize the population pharmacokinetics and the relationship between the pharmacokinetic and safety profiles of RBP-7000, a new, long-acting, sustained-release formulation of risperidone. J Clin Pharmacol. 2013;53:1010–9.
Acknowledgments
The authors would like to acknowledge Bradley Vince, DO, President and Medical Director at Vince & Associates (10103 Metcalf Avenue, Overland Park, KS 66212, USA) for patient recruitment in this study.
Conflict of Interest/Disclosure
At the time this manuscript was submitted for publication, A.F. Nasser, C. Heidbreder, P.J. Fudala and B. Zheng were full-time employees of Reckitt Benckiser Pharmaceuticals Inc. M.K. Greenwald was a full-time employee of Wayne State University, and was a paid consultant for Reckitt Benckiser Pharmaceuticals Inc. R. Gomeni was a paid consultant for Reckitt Benckiser Pharmaceuticals Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasser, A.F., Heidbreder, C., Gomeni, R. et al. A Population Pharmacokinetic and Pharmacodynamic Modelling Approach to Support the Clinical Development of RBP-6000, a New, Subcutaneously Injectable, Long-Acting, Sustained-Release Formulation of Buprenorphine, for the Treatment of Opioid Dependence. Clin Pharmacokinet 53, 813–824 (2014). https://doi.org/10.1007/s40262-014-0155-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0155-0