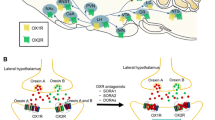
Abstract
Addiction is a chronic relapsing disorder which presents a significant global health burden and unmet medical need. The orexin/hypocretin system is an attractive potential therapeutic target as demonstrated by the successful clinical trials of antagonist medications like Suvorexant for insomnia. It is composed of two neuropeptides, orexin-A and orexin-B and two excitatory and promiscuous G-protein coupled receptors, OX1 and OX2. Orexins are known to have a variety of functions, most notably in regulating arousal, appetite and reward. The orexins have been shown to have a role in mediating the effects of several drugs of abuse, such as cocaine, morphine and alcohol via projections to key brain regions such as the ventral tegmental area, nucleus accumbens and prefrontal cortex. However, it has not yet been demonstrated whether the dual orexin receptor antagonists (DORAs) under development for insomnia are ideal drugs for the treatment of addiction. The question of whether to use a DORA or single orexin receptor antagonist (SORA) for the treatment of addiction is a key question that will need to be answered in order to maximize the clinical utility of orexin receptor antagonists. This review will examine the role of the orexin/hypocretin system in addiction, orexin-based pharmacotherapies under development and factors affecting the selection of one or both orexin receptors as drug targets for the treatment of addiction.

Similar content being viewed by others
Notes
The number needed to treat (NNT) is calculated as 1/risk difference. An increase in abstinence rate from 25 to 38 % gives a risk difference of 13 % and an NNT of 8.
References
World Health Organization. International statistical classification of diseases and related health problems (10th revision). Geneva: World Health Organization; 1992.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. vol (DSM-5™). Arlington: American Psychiatric Association; 2013.
Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1564–74. doi:10.1016/S0140-6736(13)61530-5.
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–86. doi:10.1016/S0140-6736(13)61611-6.
Pierce RC, O’Brien CP, Kenny PJ, Vanderschuren LJMJ. Rational development of addiction pharmacotherapies: successes, failures, and prospects. Cold Spring Harb Perspect Med 2012;2(6). doi:10.1101/cshperspect.a012880.
Johnson BA, Ait-Daoud N, Wang X, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338–46. doi:10.1001/jamapsychiatry.2013.2295.
Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689–98. doi:10.1038/npp.2012.14.
Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376(9752):1558–65. doi:10.1016/s0140-6736(10)61462-6.
Spanagel R, Vengeliene V, Jandeleit B, Fischer W-N, Grindstaff K, Zhang X, et al. Acamprosate produces its anti-relapse effects via calcium. Neuropsychopharmacology. 2014;39(4):783–91. doi:10.1038/npp.2013.264.
Dupouy J, Fournier J-P, Jouanjus É, Palmaro A, Poutrain J-C, Oustric S, et al. Baclofen for alcohol dependence in France: incidence of treated patients and prescription patterns—a cohort study. Eur Neuropsychopharmacol. 2014;24(2):192–9. doi:10.1016/j.euroneuro.2013.09.008.
Liu J, Wang L-N. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2013;2013(2). doi:10.1002/14651858.CD008502.pub3.
de Lecea L, Kilduff TS, Peyron C, Gao X-B, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci. 1998;95(1):322–7. doi:10.1073/pnas.95.1.322.
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–85. doi:10.1016/s0092-8674(00)80949-6.
Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev. 2012;64(3):389–420. doi:10.1124/pr.111.005546.
Sakurai T, Moriguchi T, Furuya K, Kajiwara N, Nakamura T, Yanagisawa M, et al. Structure and function of human prepro-orexin gene. J Biol Chem. 1999;274(25):17771–6. doi:10.1074/jbc.274.25.17771.
Heifetz A, Barker O, Morris GB, Law RJ, Slack M, Biggin PC. Toward an understanding of agonist binding to human Orexin-1 and Orexin-2 receptors with G-protein-coupled receptor modeling and site-directed mutagenesis. Biochemistry. 2013;52(46):8246–60. doi:10.1021/bi401119m.
Xu TR, Ward RJ, Pediani JD, Milligan G. The orexin OX1 receptor exists predominantly as a homodimer in the basal state: potential regulation of receptor organization by both agonist and antagonist ligands. Biochem J. 2011;439(1):171–83. doi:10.1042/bj20110230.
Jäntti MH, Mandrika I, Kukkonen JP. Human orexin/hypocretin receptors form constitutive homo- and heteromeric complexes with each other and with human CB1 cannabinoid receptors. Biochem Biophys Res Commun. 2014;445(2):486–90. doi:10.1016/j.bbrc.2014.02.026.
Ward RJ, Pediani JD, Milligan G. Heteromultimerization of cannabinoid CB1 receptor and orexin OX1 receptor generates a unique complex in which both protomers are regulated by orexin A. J Biol Chem. 2011;286(43):37414–28. doi:10.1074/jbc.M111.287649.
Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21(19):RC168.
Furutani N, Hondo M, Kageyama H, Tsujino N, Mieda M, Yanagisawa M, et al. Neurotensin co-expressed in orexin-producing neurons in the lateral hypothalamus plays an important role in regulation of sleep/wakefulness states. PLoS One. 2013;8(4):e62391. doi:10.1371/journal.pone.0062391.
Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465(4):593–603. doi:10.1002/cne.10860.
Henny P, Brischoux F, Mainville L, Stroh T, Jones BE. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience. 2010;169(3):1150–7. doi:10.1016/j.neuroscience.2010.06.003.
Harthoorn LF, Sañé A, Nethe M, Heerikhuize JJ. Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell Mol Neurobiol. 2005;25(8):1209–23. doi:10.1007/s10571-005-8184-8.
Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, et al. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther. 2003;305(2):507–14. doi:10.1124/jpet.102.048025.
Holmqvist T, Johansson L, Östman M, Ammoun S, Åkerman KEO, Kukkonen JP. OX1 orexin receptors couple to adenylyl cyclase regulation via multiple mechanisms. J Biol Chem. 2005;280(8):6570–9. doi:10.1074/jbc.M407397200.
Zhu Y, Miwa Y, Yamanaka A, Yada T, Shibahara M, Abe Y, et al. Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci. 2003;92(3):259–66. doi:10.1254/jphs.92.259.
Smart D, Jerman JC, Brough SJ, Rushton SL, Murdock PR, Jewitt F, et al. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol. 1999;128(1):1–3. doi:10.1038/sj.bjp.0702780.
Magga J, Bart G, Oker-Blom C, Kukkonen JP, Åkerman KEO, Näsman J. Agonist potency differentiates G protein activation and Ca2+ signalling by the orexin receptor type 1. Biochem Pharmacol. 2006;71(6):827–36. doi:10.1016/j.bcp.2005.12.021.
Johansson L, Ekholm ME, Kukkonen JP. Regulation of OX1 orexin/hypocretin receptor-coupling to phospholipase C by Ca2+ influx. Br J Pharmacol. 2007;150(1):97–104. doi:10.1038/sj.bjp.0706959.
Lund P-E, Shariatmadari R, Uustare A, Detheux M, Parmentier M, Kukkonen JP, et al. The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J Biol Chem. 2000;275(40):30806–12. doi:10.1074/jbc.M002603200.
Näsman J, Bart G, Larsson K, Louhivuori L, Peltonen H, Åkerman KEO. The orexin OX1 receptor regulates Ca2+ entry via diacylglycerol-activated channels in differentiated neuroblastoma cells. J Neurosci. 2006;26(42):10658–66. doi:10.1523/jneurosci.2609-06.2006.
Peltonen HM, Magga JM, Bart G, Turunen PM, Antikainen MSH, Kukkonen JP, et al. Involvement of TRPC3 channels in calcium oscillations mediated by OX1 orexin receptors. Biochem Biophys Res Commun. 2009;385(3):408–12. doi:10.1016/j.bbrc.2009.05.077.
Jäntti MH, Putula J, Somerharju P, Frohman MA, Kukkonen JP. OX1 orexin/hypocretin receptor activation of phospholipase D. Br J Pharmacol. 2012;165(4b):1109–23. doi:10.1111/j.1476-5381.2011.01565.x.
Turunen PM, Ekholm ME, Somerharju P, Kukkonen JP. Arachidonic acid release mediated by OX1 orexin receptors. Br J Pharmacol. 2010;159(1):212–21. doi:10.1111/j.1476-5381.2009.00535.x.
Turunen PM, Jäntti MH, Kukkonen JP. OX1 orexin/hypocretin receptor signaling through arachidonic acid and endocannabinoid release. Mol Pharmacol. 2012;82(2):156–67. doi:10.1124/mol.112.078063.
Tang J, Chen J, Ramanjaneya M, Punn A, Conner AC, Randeva HS. The signalling profile of recombinant human orexin-2 receptor. Cell Signal. 2008;20(9):1651–61. doi:10.1016/j.cellsig.2008.05.010.
Karteris E, Randeva HS, Grammatopoulos DK, Jaffe RB, Hillhouse EW. Expression and coupling characteristics of the CRH and orexin type 2 receptors in human fetal adrenals. J Clin Endocrinol Metab. 2001;86(9):4512–9. doi:10.1210/jc.86.9.4512.
Urbańska A, Sokołowska P, Woldan-Tambor A, Biegańska K, Brix B, Jöhren O, et al. Orexins/hypocretins acting at Gi protein-coupled OX2 receptors inhibit cyclic AMP synthesis in the primary neuronal cultures. J Mol Neurosci. 2012;46(1):10–7. doi:10.1007/s12031-011-9526-2.
Hoang QV, Bajic D, Yanagisawa M, Nakajima S, Nakajima Y. Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol. 2003;90(2):693–702. doi:10.1152/jn.00001.2003.
Dalrymple MB, Jaeger WC, Eidne KA, Pfleger KDG. Temporal profiling of orexin receptor-arrestin-ubiquitin complexes reveals differences between receptor subtypes. J Biol Chem. 2011;286(19):16726–33. doi:10.1074/jbc.M111.223537.
Jaeger WC, Seeber RM, Eidne KA, Pfleger KDG. Molecular determinants of orexin receptor-arrestin-ubiquitin complex formation. Br J Pharmacol. 2014;171(2):364–74. doi:10.1111/bph.12481.
Darker JG, Porter RA, Eggleston DS, Smart D, Brough SJ, Sabido-David C, et al. Structure-activity analysis of truncated orexin-A analogues at the orexin-1 receptor. Bioorg Med Chem Lett. 2001;11(5):737–40. doi:10.1016/S0960-894X(01)00043-9.
Lang M, Söll RM, Dürrenberger F, Dautzenberg FM, Beck-Sickinger AG. Structure-activity studies of orexin A and orexin B at the human orexin 1 and orexin 2 receptors led to orexin 2 receptor selective and orexin 1 receptor preferring ligands. J Med Chem. 2004;47(5):1153–60. doi:10.1021/jm030982t.
German NA, Decker AM, Gilmour BP, Thomas BF, Zhang Y. Truncated orexin peptides: structure-activity relationship studies. ACS Med Chem Lett. 2013;4(12):1224–7. doi:10.1021/ml400333a.
Asahi S, Egashira S-I, Matsuda M, Iwaasa H, Kanatani A, Ohkubo M, et al. Development of an orexin-2 receptor selective agonist, [Ala11, d-Leu15]orexin-B. Bioorg Med Chem Lett. 2003;13(1):111–3. doi:10.1016/S0960-894X(02)00851-X.
Putula J, Turunen PM, Johansson L, Näsman J, Ra R, Korhonen L, et al. Orexin/hypocretin receptor chimaeras reveal structural features important for orexin peptide distinction. FEBS Lett. 2011;585(9):1368–74. doi:10.1016/j.febslet.2011.04.020.
Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, et al. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett. 2001;11(14):1907–10. doi:10.1016/S0960-894X(01)00343-2.
Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, et al. SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol. 2001;132(6):1179–82. doi:10.1038/sj.bjp.0703953.
Langmead CJ, Jerman JC, Brough SJ, Scott C, Porter RA, Herdon HJ. Characterisation of the binding of [3H]-SB-674042, a novel nonpeptide antagonist, to the human orexin-1 receptor. Br J Pharmacol. 2004;141(2):340–6. doi:10.1038/sj.bjp.0705610.
Steiner MA, Gatfield J, Brisbare-Roch C, Dietrich H, Treiber A, Jenck F, et al. Discovery and characterization of ACT-335827, an orally available, brain penetrant orexin receptor type 1 selective antagonist. ChemMedChem. 2013;8(6):898–903. doi:10.1002/cmdc.201300003.
Steiner MA, Sciarretta C, Pasquali A, Jenck F. The selective orexin receptor 1 antagonist ACT-335827 in a rat model of diet-induced obesity associated with metabolic syndrome. Front Pharmacol. 2013;4. doi:10.3389/fphar.2013.00165.
Hirose M, Egashira S-I, Goto Y, Hashihayata T, Ohtake N, Iwaasa H, et al. N-acyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline: the first orexin-2 receptor selective non-peptidic antagonist. Bioorg Med Chem Lett. 2003;13(24):4497–9. doi:10.1016/j.bmcl.2003.08.038.
McAtee LC, Sutton SW, Rudolph DA, Li X, Aluisio LE, Phuong VK, et al. Novel substituted 4-phenyl-[1,3]dioxanes: potent and selective orexin receptor 2 (OX2R) antagonists. Bioorg Med Chem Lett. 2004;14(16):4225–9. doi:10.1016/j.bmcl.2004.06.032.
Malherbe P, Borroni E, Gobbi L, Knust H, Nettekoven M, Pinard E, et al. Biochemical and behavioural characterization of EMPA, a novel high-affinity, selective antagonist for the OX2 receptor. Br J Pharmacol. 2009;156(8):1326–41. doi:10.1111/j.1476-5381.2009.00127.x.
Fitch TE, Benvenga MJ, Jesudason CD, Zink C, Vandergriff AB, Menezes M, et al. LSN2424100: a novel, potent orexin-2 receptor antagonist with selectivity over orexin-1 receptors and activity in an animal model predictive of antidepressant-like efficacy. Front Neurosci. 2014;8. doi:10.3389/fnins.2014.00005.
Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13(2):150–5. doi:10.1038/nm1544.
Hoever P, de Haas S, Winkler J, Schoemaker RC, Chiossi E, van Gerven J, et al. Orexin receptor antagonism, a new sleep-promoting paradigm: an ascending single-dose study with almorexant. Clin Pharmacol Ther. 2010;87(5):593–600. doi:10.1038/clpt.2010.19.
Hoever P, de Haas SL, Dorffner G, Chiossi E, van Gerven JM, Dingemanse J. Orexin receptor antagonism: an ascending multiple-dose study with almorexant. J Psychopharmacol. 2012;26(8):1071–80. doi:10.1177/0269881112448946.
Hoever P, Dorffner G, Benes H, Penzel T, Danker-Hopfe H, Barbanoj MJ, et al. Orexin receptor antagonism, a new sleep-enabling paradigm: a proof-of-concept clinical trial. Clin Pharmacol Ther. 2012;91(6):975–85. doi:10.1038/clpt.2011.370.
Heifetz A, Morris GB, Biggin PC, Barker O, Fryatt T, Bentley J, et al. Study of human Orexin-1 and -2 G-protein-coupled receptors with novel and published antagonists by modeling, molecular dynamics simulations, and site-directed mutagenesis. Biochemistry. 2012;51(15):3178–97. doi:10.1021/bi300136h.
Actelion Pharmaceuticals, GlaxoSmithKline. Actelion and GSK discontinue clinical development of almorexant. Allschwil/Basel, Switzerland and London. http://www.actelion.com; 2011. Accessed 21 Dec 2013.
Cruz HG, Hoever P, Chakraborty B, Schoedel K, Sellers EM, Dingemanse J. Assessment of the abuse liability of a dual orexin receptor antagonist: a crossover study of almorexant and zolpidem in recreational drug users. CNS Drugs. 2014;28(4):361–72. doi:10.1007/s40263-014-0150-x.
Shakeri-Nejad K, Hoch M, Hoever P, Dingemanse J. Influence of mild and moderate liver impairment on the pharmacokinetics and metabolism of almorexant, a dual orexin receptor antagonist. Eur J Pharm Sci. 2013;49(5):836–44. doi:10.1016/j.ejps.2013.06.002.
Perrey DA, German NA, Gilmour BP, Li J-X, Harris DL, Thomas BF, et al. Substituted tetrahydroisoquinolines as selective antagonists for the orexin 1 receptor. J Med Chem. 2013;56(17):6901–16. doi:10.1021/jm400720h.
Di Fabio R, Pellacani A, Faedo S, Roth A, Piccoli L, Gerrard P, et al. Discovery process and pharmacological characterization of a novel dual orexin 1 and orexin 2 receptor antagonist useful for treatment of sleep disorders. Bioorg Med Chem Lett. 2011;21(18):5562–7. doi:10.1016/j.bmcl.2011.06.086.
Bettica P, Nucci G, Pyke C, Squassante L, Zamuner S, Ratti E, et al. Phase I studies on the safety, tolerability, pharmacokinetics and pharmacodynamics of SB-649868, a novel dual orexin receptor antagonist. J Psychopharmacol. 2012;26(8):1058–70. doi:10.1177/0269881111408954.
GlaxoSmithKline. A clinical study to evaluate the pharmacokinetic profile of SB-649868 in elderly and female population. clinicaltrials.gov. 2010. clinicaltrials.gov/show/NCT00534872. Accessed 22 Dec 2013.
Roecker AJ, Mercer SP, Schreier JD, Cox CD, Fraley ME, Steen JT, et al. Discovery of 5″-chloro-N-[(5,6-dimethoxypyridin-2-yl)methyl]-2,2′:5′,3″-terpyridine-3′-carboxamide (MK-1064): a selective orexin 2 receptor antagonist (2-SORA) for the treatment of insomnia. ChemMedChem. 2014;9(2):311–22. doi:10.1002/cmdc.201300447.
Coleman PJ, Schreier JD, Cox CD, Breslin MJ, Whitman DB, Bogusky MJ, et al. Discovery of [(2R,5R)-5-{[(5-fluoropyridin-2-yl)oxy]methyl}-2-methylpiperidin-1-yl][5-methyl-2-(pyrimidin-2-yl)phenyl]methanone (MK-6096): a dual orexin receptor antagonist with potent sleep-promoting properties. ChemMedChem. 2012;7(3):415–24. doi:10.1002/cmdc.201200025.
Winrow CJ, Gotter AL, Cox CD, Tannenbaum PL, Garson SL, Doran SM, et al. Pharmacological characterization of MK-6096—a dual orexin receptor antagonist for insomnia. Neuropharmacology. 2012;62(2):978–87. doi:10.1016/j.neuropharm.2011.10.003.
Merck Sharp & Dohme. Polysomnography study of MK6096 in patients with primary insomnia (6096-011). clinicaltrials.gov. 2011. clinicaltrials.gov/show/NCT01021852. Accessed 19 Mar 2014.
Merck Sharp & Dohme. A study of the safety and efficacy of MK-6096 for migraine prophylaxis in participants with episodic migraine (MK-6096-020). clinicaltrials.gov. 2012. clinicaltrials.gov/show/NCT01513291. Accessed 19 Mar 2014.
Merck Sharp & Dohme. Study to evaluate MK-6096 in the treatment of painful diabetic neuropathy (PDN) in adults (MK-6096-021 AM1). clinicaltrials.gov. 2013. clinicaltrials.gov/show/NCT01564459. Accessed 19 Mar 2014.
Merck Sharp & Dohme. Safety and efficacy of MK-6096 as adjunctive therapy in participants with major depressive disorder and partial response to antidepressant monotherapy (MK-6096-022 AM3). clinicaltrials.gov. 2013. clinicaltrials.gov/show/NCT01554176. Accessed 19 Mar 2014.
Merck. Merck receives complete response letter for Suvorexant, Merck’s investigational medicine for insomnia. Whitehouse Station, N.J. http://www.mercknewsroom.com; 2013. Accessed 21 Dec 2013.
Cox CD, Breslin MJ, Whitman DB, Schreier JD, McGaughey GB, Bogusky MJ, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53(14):5320–32. doi:10.1021/jm100541c.
Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, et al. Promotion of sleep by suvorexant—a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1–2):52–61. doi:10.3109/01677063.2011.566953.
Herring WJMDP, Snyder EP, Budd KBS, Hutzelmann JMS, Snavely DMA, Liu KP, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265–74. doi:10.1212/WNL.0b013e31827688ee.
Sun H, Kennedy WP, Wilbraham D, Lewis N, Calder N, Li X, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36(2):259–67. doi:10.5665/sleep.2386.
Allard JS, Tizabi Y, Shaffery JP, Ovid Trouth C, Manaye K. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides. 2004;38(5):311–5. doi:10.1016/j.npep.2004.06.004.
Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–74. doi:10.1016/S0896-6273(00)00058-1.
Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep. 2009;32(8):993–8.
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015.
Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-β-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464(2):220–37. doi:10.1002/cne.10783.
Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827(1–2):243–60. doi:10.1016/S0006-8993(99)01336-0.
Schmitt O, Usunoff KG, Lazarov NE, Itzev DE, Eipert P, Rolfs A, et al. Orexinergic innervation of the extended amygdala and basal ganglia in the rat. Brain Struct Funct. 2012;217(2):233–56. doi:10.1007/s00429-011-0343-8.
Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–87. doi:10.1016/s0306-4522(02)00017-9.
Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. doi:10.1002/cne.1190.
Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LHT, Guan X-M. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438(1–2):71–5. doi:10.1016/s0014-5793(98)01266-6.
Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103(3):777–97. doi:10.1016/s0306-4522(01)00033-1.
Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104(1–3):131–44. doi:10.1016/s0167-0115(01)00357-3.
Martin G, Fabre V, Siggins GR, de Lecea L. Interaction of the hypocretins with neurotransmitters in the nucleus accumbens. Regul Pept. 2002;104(1–3):111–7. doi:10.1016/s0167-0115(01)00354-8.
Porkka-Heiskanen T, Alanko L, Kalinchuk A, Heiskanen S, Stenberg D. The effect of age on prepro-orexin gene expression and contents of orexin A and B in the rat brain. Neurobiol Aging. 2004;25(2):231–8. doi:10.1016/S0197-4580(03)00043-5.
Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One. 2011;6(5):e20360. doi:10.1371/journal.pone.0020360.
Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–13. doi:10.1016/S0896-6273(03)00331-3.
Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24(46):10493–501. doi:10.1523/jneurosci.3171-04.2004.
Muschamp JW, Dominguez JM, Sato SM, Shen R-Y, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27(11):2837–45. doi:10.1523/jneurosci.4121-06.2007.
Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, et al. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23(8):3106–11.
Laorden ML, Ferenczi S, Pintér-Kübler B, González-Martín LL, Lasheras MC, Kovács KJ, et al. Hypothalamic orexin—a neurons are involved in the response of the brain stress system to morphine withdrawal. PLoS One. 2012;7(5):e36871. doi:10.1371/journal.pone.0036871.
Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–9. doi:10.1038/nature04071.
Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004;24(12):2923–33. doi:10.1523/jneurosci.5282-03.2004.
Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and γ-aminobutyric acid neurons. J Comp Neurol. 2007;503(5):668–84. doi:10.1002/cne.21420.
Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26(2):398–405. doi:10.1523/jneurosci.2761-05.2006.
Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23(1):7–11.
Li Y, van den Pol AN. μ-Opioid receptor-mediated depression of the hypothalamic hypocretin/orexin arousal system. J Neurosci. 2008;28(11):2814–9. doi:10.1523/jneurosci.5447-07.2008.
Richardson KA, Aston-Jones G. Lateral hypothalamic orexin/hypocretin neurons that project to ventral tegmental area are differentially activated with morphine preference. J Neurosci. 2012;32(11):3809–17. doi:10.1523/jneurosci.3917-11.2012.
Smith RJ, Aston-Jones G. Orexin / hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur J Neurosci. 2012;35(5):798–804. doi:10.1111/j.1460-9568.2012.08013.x.
Sharf R, Sarhan M, DiLeone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64(3):175–83. doi:10.1016/j.biopsych.2008.03.006.
Schmeichel BE, Vendruscolo LF, Misra KK, Schlosburg JE, Contet C, Grigoriadis DE, et al. Hypocretin-2 receptor antagonism dose-dependently reduces compulsive-like self-administration of heroin in rats allowed extended access. Neuroscience 2013. San Diego: Society for Neuroscience; 2013.
Steiner MA, Lecourt H, Jenck F. The dual orexin receptor antagonist almorexant, alone and in combination with morphine, cocaine and amphetamine, on conditioned place preference and locomotor sensitization in the rat. Int J Neuropsychopharmacol. 2013;16:417–32. doi:10.1017/S1461145712000193.
Lawrence AJ, Cowen MS, Yang H-J, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148(6):752–9. doi:10.1038/sj.bjp.0706789.
Martin-Fardon R, Weiss F. Blockade of hypocretin receptor-1 preferentially prevents cocaine seeking: comparison with natural reward seeking. Neuroreport. 2014;25(7):485–8. doi:10.1097/WNR.0000000000000120.
Richards J, Simms J, Steensland P, Taha S, Borgland S, Bonci A, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology. 2008;199(1):109–17. doi:10.1007/s00213-008-1136-5.
Anderson RI, Becker HC, Adams BL, Jesudason CD, Rorick-Kehn LM. Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Front Neurosci. 2014;8. doi:10.3389/fnins.2014.00033.
Shoblock J, Welty N, Aluisio L, Fraser I, Motley S, Morton K, et al. Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology. 2011;215(1):191–203. doi:10.1007/s00213-010-2127-x.
Jupp B, Krstew E, Dezsi G, Lawrence AJ. Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin1 receptors. Br J Pharmacol. 2011;162(4):880–9. doi:10.1111/j.1476-5381.2010.01088.x.
Brown RM, Khoo SY-S, Lawrence AJ. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol. 2013;16(9):2067–79. doi:10.1017/S1461145713000333.
Sharma R, Sahota P, Thakkar MM. Role of adenosine and the orexinergic perifornical hypothalamus in sleep-promoting effects of ethanol. Sleep. 2014;37(3):525–33. doi:10.5665/sleep.3490.
Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63(2):152–7. doi:10.1016/j.biopsych.2007.02.002.
Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, et al. The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration. PLoS One. 2012;7(9):e44726. doi:10.1371/journal.pone.0044726.
Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58(1):179–84. doi:10.1016/j.neuropharm.2009.06.042.
Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102(52):19168–73. doi:10.1073/pnas.0507480102.
Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30(3):493–503. doi:10.1111/j.1460-9568.2009.06844.x.
Rao Y, Mineur YS, Gan G, Wang AH, Liu Z-W, Wu X, et al. Repeated in vivo exposure of cocaine induces long-lasting synaptic plasticity in hypocretin/orexin-producing neurons in the lateral hypothalamus in mice. J Physiol. 2013;591(7):1951–66. doi:10.1113/jphysiol.2012.246983.
Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003;28(1):73–6. doi:10.1038/sj.npp.1300011.
Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601. doi:10.1016/j.neuron.2006.01.016.
Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci. 2008;28(8):1545–56. doi:10.1111/j.1460-9568.2008.06397.x.
Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2013;226(4):687–98. doi:10.1007/s00213-012-2681-5.
Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. J Neurosci. 2010;30(46):15585–99. doi:10.1523/jneurosci.2871-10.2010.
España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR. The hypocretin–orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31(2):336–48. doi:10.1111/j.1460-9568.2009.07065.x.
Hollander J, Pham D, Fowler C, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: Pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6. doi:10.3389/fnbeh.2012.00047.
Yeoh JW, James MH, Jobling P, Bains JS, Graham BA, Dayas CV. Cocaine potentiates excitatory drive in the perifornical/lateral hypothalamus. J Physiol. 2012;590(16):3677–89. doi:10.1113/jphysiol.2012.230268.
Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, et al. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J Pharmacol Exp Ther. 2012;340(3):801–9. doi:10.1124/jpet.111.187567.
Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV. Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Front Neurosci. 2014;8:36. doi:10.3389/fnins.2014.00036.
Zhou L, Smith RJ, Do PH, Aston-Jones G, See RE. Repeated orexin 1 receptor antagonism effects on cocaine seeking in rats. Neuropharmacology. 2012;63(7):1201–7. doi:10.1016/j.neuropharm.2012.07.044.
Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci. 2008;105(49):19480–5. doi:10.1073/pnas.0808023105.
Plaza-Zabala A, Flores A, Martin-Garcia E, Saravia R, Maldonado R, Berrendero F. A role for hypocretin/orexin receptor-1 in cue-induced reinstatement of nicotine-seeking behavior. Neuropsychopharmacology. 2013;38(9):1724–36. doi:10.1038/npp.2013.72.
Plaza-Zabala A, Martín-García E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30(6):2300–10. doi:10.1523/jneurosci.5724-09.2010.
Plaza-Zabala A, Flores Á, Maldonado R, Berrendero F. Hypocretin/orexin signaling in the hypothalamic paraventricular nucleus is essential for the expression of nicotine withdrawal. Biol Psychiatry. 2012;71(3):214–23. doi:10.1016/j.biopsych.2011.06.025.
Flores Á, Maldonado R, Berrendero F. The hypocretin/orexin receptor-1 as a novel target to modulate cannabinoid reward. Biol Psychiatry. 2014;75(6):499–507. doi:10.1016/j.biopsych.2013.06.012.
Cristino L, Busetto G, Imperatore R, Ferrandino I, Palomba L, Silvestri C, et al. Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc Natl Acad Sci. 2013;110(24):E2229–38. doi:10.1073/pnas.1219485110.
Rotter A, Bayerlein K, Hansbauer M, Weiland J, Sperling W, Kornhuber J, et al. Orexin A expression and promoter methylation in patients with cannabis dependence in comparison to nicotine-dependent cigarette smokers and nonsmokers. Neuropsychobiology. 2012;66(2):126–33. doi:10.1159/000339457.
Vittoz NM, Schmeichel B, Berridge CW. Hypocretin /orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur J Neurosci. 2008;28(8):1629–40. doi:10.1111/j.1460-9568.2008.06453.x.
Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do μ-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25(50):11777–86. doi:10.1523/jneurosci.2329-05.2005.
Peciña S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered ‘wanting’ for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci. 2013;37(9):1529–40. doi:10.1111/ejn.12174.
Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci. 2014;34(12):4239–50. doi:10.1523/jneurosci.4458-13.2014.
Carelli RM, Wondolowski J. Anatomic distribution of reinforcer selective cell firing in the core and shell of the nucleus accumbens. Synapse. 2006;59(2):69–73. doi:10.1002/syn.20217.
Robinson DL, Carelli RM. Distinct subsets of nucleus accumbens neurons encode operant responding for ethanol versus water. Eur J Neurosci. 2008;28(9):1887–94. doi:10.1111/j.1460-9568.2008.06464.x.
Cameron CM, Carelli RM. Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. Eur J Neurosci. 2012;35(6):940–51. doi:10.1111/j.1460-9568.2012.08024.x.
Patyal R, Woo EY, Borgland SL. Local hypocretin-1 modulates terminal dopamine concentration in the nucleus accumbens shell. Front Behav Neurosci. 2012;6. doi:10.3389/fnbeh.2012.00082.
Ho C-Y, Berridge KC. An orexin hotspot in ventral pallidum amplifies hedonic ‘liking’ for sweetness. Neuropsychopharmacology. 2013;38(9):1655–64. doi:10.1038/npp.2013.62.
Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146(1–2):145–57. doi:10.1016/j.bbr.2003.09.023.
McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–63.
Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168(1–2):66–74. doi:10.1007/s00213-002-1283-z.
McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168(1–2):57–65. doi:10.1007/s00213-002-1196-x.
Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37(2):259–68. doi:10.1111/ejn.12031.
Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2005;31(2):384–95. doi:10.1038/sj.npp.1300807.
Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–19. doi:10.1093/cercor/10.3.206.
Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. NeuroImage. 2012;60(1):252–62. doi:10.1016/j.neuroimage.2011.12.024.
Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059(2):179–88. doi:10.1016/j.brainres.2005.08.035.
Pasumarthi RK, Fadel J. Activation of orexin/hypocretin projections to basal forebrain and paraventricular thalamus by acute nicotine. Brain Res Bull. 2008;77(6):367–73. doi:10.1016/j.brainresbull.2008.09.014.
Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience. 2012;210:243–8. doi:10.1016/j.neuroscience.2012.02.036.
Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic–thalamic–striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493(1):72–85. doi:10.1002/cne.20769.
Parsons MP, Li S, Kirouac GJ. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse. 2006;59(8):480–90. doi:10.1002/syn.20264.
James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, et al. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14(05):684–90. doi:10.1017/S1461145711000423.
Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res. 2010;1314:145–61. doi:10.1016/j.brainres.2009.12.027.
Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. Changes in emotional behavior produced by orexin microinjections in the paraventricular nucleus of the thalamus. Pharmacol Biochem Behav. 2010;95(1):121–8. doi:10.1016/j.pbb.2009.12.016.
Li Y, Wang H, Qi K, Chen X, Li S, Sui N, et al. Orexins in the midline thalamus are involved in the expression of conditioned place aversion to morphine withdrawal. Physiol Behav. 2011;102(1):42–50. doi:10.1016/j.physbeh.2010.10.006.
James MH, Dayas CV. What about me…? The PVT: A role for the paraventricular thalamus (PVT) in drug-seeking behaviour. Front Behav Neurosci. 2013;7:18. doi:10.3389/fnbeh.2013.00018.
Jupp B, Krivdic B, Krstew E, Lawrence AJ. The orexin1 receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res. 2011;1391:54–9. doi:10.1016/j.brainres.2011.03.045.
Martin-Fardon R, Weiss F. N-(2-methyl-6-benzoxazolyl)-N′-1,5-naphthyridin-4-yl urea (SB334867), a hypocretin receptor-1 antagonist, preferentially prevents ethanol seeking: comparison with natural reward seeking. Addict Biol. 2014;19(2):233–6. doi:10.1111/j.1369-1600.2012.00480.x.
Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146(2):525–36. doi:10.1016/j.neuroscience.2007.01.063.
Hamlin AS, Blatchford KE, McNally GP. Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience. 2006;143(1):25–38. doi:10.1016/j.neuroscience.2006.07.035.
Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology. 2013;226(1):155–65. doi:10.1007/s00213-012-2902-y.
Cason AM, Aston-Jones G. Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharmacology. 2013;228(3):499–507. doi:10.1007/s00213-013-3051-7.
Borgland SL, Chang S-J, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29(36):11215–25. doi:10.1523/jneurosci.6096-08.2009.
Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol—preferring Sprague-Dawley rats. Alcohol. 2009;43(5):379–86. doi:10.1016/j.alcohol.2009.07.002.
Brown RM, Lawrence AJ. Ascending orexinergic pathways and alcohol-seeking. Curr Opin Neurobiol. 2013;23(4):467–72. doi:10.1016/j.conb.2013.02.014.
Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 2005;1050(1–2):156–62. doi:10.1016/j.brainres.2005.05.045.
Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology. 2005;182(1):75–83. doi:10.1007/s00213-005-0040-5.
Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330(1):142–51. doi:10.1124/jpet.109.152009.
Morairty SR, Revel FG, Malherbe P, Moreau J-L, Valladao D, Wettstein JG, et al. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS One. 2012;7(7):e39131. doi:10.1371/journal.pone.0039131.
Russell SH, Small CJ, Kennedy AR, Stanley SA, Seth A, Murphy KG, et al. Orexin A interactions in the hypothalamo-pituitary gonadal axis. Endocrinology. 2001;142(12):5294–302. doi:10.1210/endo.142.12.8558.
Hoch M, Hoever P, Alessi F, Marjason J, Dingemanse J. Pharmacokinetics and tolerability of almorexant in Japanese and Caucasian healthy male subjects. Pharmacology. 2011;88(3–4):121–6. doi:10.1159/000330098.
Hoever P, Hay J, Rad M, Cavallaro M, van Gerven JM, Dingemanse J. Tolerability, pharmacokinetics, and pharmacodynamics of single-dose almorexant, an orexin receptor antagonist, in healthy elderly subjects. J Clin Psychopharmacol. 2013;33(3):363–70. doi:10.1097/JCP.0b013e31828f5a7a.
Hoyer D, Dürst T, Fendt M, Jacobson LH, Betschart C, Hintermann S, et al. Distinct effects of IPSU and suvorexant on mouse sleep architecture. Front Neurosci. 2013;7. doi:10.3389/fnins.2013.00235.
Sifferlen T, Koberstein R, Cottreel E, Boller A, Weller T, Gatfield J, et al. Structure-activity relationship studies and sleep-promoting activity of novel 1-chloro-5,6,7,8-tetrahydroimidazo[1,5-a]pyrazine derivatives as dual orexin receptor antagonists. Part 2. Bioorg Med Chem Lett. 2013;23(13):3857–63. doi:10.1016/j.bmcl.2013.04.071.
Uslaner JM, Tye SJ, Eddins DM, Wang X, Fox SV, Savitz AT, et al. Orexin receptor antagonists differ from standard sleep drugs by promoting sleep at doses that do not disrupt cognition. Sci Transl Med. 2013;5(179):179ra44. doi:10.1126/scitranslmed.3005213.
Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28(12):3071–5. doi:10.1523/jneurosci.5584-07.2008.
Nocjar C, Zhang J, Feng P, Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–53. doi:10.1016/j.neuroscience.2012.05.033.
Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16(1):111–5. doi:10.1038/nm.2075.
Hirota K, Kushikata T, Kudo M, Kudo T, Smart D, Matsuki A. Effects of central hypocretin-1 administration on hemodynamic responses in young-adult and middle-aged rats. Brain Res. 2003;981(1–2):143–50. doi:10.1016/S0006-8993(03)03002-6.
Li A, Hindmarch CCT, Nattie EE, Paton JFR. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol. 2013;591(17):4237–48. doi:10.1113/jphysiol.2013.256271.
Ramirez AD, Gotter AL, Fox SV, Tannenbaum PL, Yao L, Tye SJ, et al. Dual orexin receptor antagonists show distinct effects on locomotor performance, ethanol interaction and sleep architecture relative to gamma-aminobutyric acid-A receptor modulators. Front Neurosci. 2013;7. doi:10.3389/fnins.2013.00254.
Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135(3):561–71. doi:10.1016/j.cell.2008.08.040.
Faedo S, Perdonà E, Antolini M, di Fabio R, Merlo Pich E, Corsi M. Functional and binding kinetic studies make a distinction between OX1 and OX2 orexin receptor antagonists. Eur J Pharmacol. 2012;692(1–3):1–9. doi:10.1016/j.ejphar.2012.07.007.
McElhinny CJ Jr, Lewin AH, Mascarella SW, Runyon S, Brieaddy L, Carroll FI. Hydrolytic instability of the important orexin 1 receptor antagonist SB-334867: possible confounding effects on in vivo and in vitro studies. Bioorg Med Chem Lett. 2012;22(21):6661–4. doi:10.1016/j.bmcl.2012.08.109.
Zhang G-C, Mao L-M, Liu X-Y, Wang JQ. Long-lasting up-regulation of orexin receptor type 2 protein levels in the rat nucleus accumbens after chronic cocaine administration. J Neurochem. 2007;103(1):400–7. doi:10.1111/j.1471-4159.2007.04748.x.
Voorhees C, Cunningham C. Involvement of the orexin/hypocretin system in ethanol conditioned place preference. Psychopharmacology. 2011;214(4):805–18. doi:10.1007/s00213-010-2082-6.
Plaza-Zabala A, Li X, Milovanovic M, Loweth JA, Maldonado R, Berrendero F, et al. An investigation of interactions between hypocretin/orexin signaling and glutamate receptor surface expression in the rat nucleus accumbens under basal conditions and after cocaine exposure. Neurosci Lett. 2013;557, Part B:101–6. doi:10.1016/j.neulet.2013.10.038.
Gozzi A, Turrini G, Piccoli L, Massagrande M, Amantini D, Antolini M, et al. Functional magnetic resonance imaging reveals different neural substrates for the effects of orexin-1 and orexin-2 receptor antagonists. PLoS One. 2011;6(1):e16406. doi:10.1371/journal.pone.0016406.
Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62(2):211–20.
O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278(5335):66–70. doi:10.1126/science.278.5335.66.
Miller WR, Manuel JK. How large must a treatment effect be before it matters to practitioners? An estimation method and demonstration. Drug Alcohol Rev. 2008;27(5):524–8. doi:10.1080/09595230801956165.
Ketter TA, Citrome L, Wang PW, Culver JL, Srivastava S. Treatments for bipolar disorder: can number needed to treat/harm help inform clinical decisions? Acta Psychiatr Scand. 2011;123(3):175–89. doi:10.1111/j.1600-0447.2010.01645.x.
Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2013;67(11):1089–104. doi:10.1111/ijcp.12298.
Citrome L. Vortioxetine for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(1):60–82. doi:10.1111/ijcp.12350.
Rösner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010;2010(9). doi:10.1002/14651858.CD004332.pub2.
Bouton ME, Winterbauer NE, Todd TP. Relapse processes after the extinction of instrumental learning: Renewal, resurgence, and reacquisition. Behav Processes. 2012;90(1):130–41. doi:10.1016/j.beproc.2012.03.004.
Delamater AR, Westbrook RF. Psychological and neural mechanisms of experimental extinction: a selective review. Neurobiol Learn Mem. 2014;108:38–51. doi:10.1016/j.nlm.2013.09.016.
Callander GE, Olorunda M, Monna D, Schuepbach E, Langenegger D, Betschart C, et al. Kinetic properties of ‘dual’ orexin receptor antagonists at OX1R and OX2R orexin receptors. Front Neurosci. 2013;7. doi:10.3389/fnins.2013.00230.
Neubig RR, Spedding M, Kenakin T, Christopoulos A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol Rev. 2003;55(4):597–606. doi:10.1124/pr.55.4.4.
Acknowledgements
RMB and SYK are supported by the National Health and Medical Research Council of Australia. SYK is supported by an Australian Postgraduate Award; RMB is supported by a Peter Doherty Fellowship. The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khoo, S.YS., Brown, R.M. Orexin/Hypocretin Based Pharmacotherapies for the Treatment of Addiction: DORA or SORA?. CNS Drugs 28, 713–730 (2014). https://doi.org/10.1007/s40263-014-0179-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0179-x