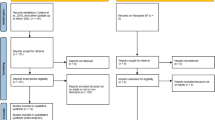
Abstract
Stimulants (methylphenidate or amphetamines) are the recommended first-line option for the pharmacological treatment of individuals with attention deficit hyperactivity disorder (ADHD). However, some patients with ADHD will not respond optimally to stimulants. Here, we discuss strategies to manage stimulant-refractory ADHD, based on the recommendations advanced in clinical guidelines, knowledge of expert practice in the field, and our own clinical recommendations, informed by a comprehensive literature search in PubMed, PsycInfo, EMBASE + EMBASE classic, OVID Medline, and Web of Science (up to 30 March 2021). We first highlight the importance of stimulant optimization as an effective strategy to increase response. We then discuss a series of factors that should be considered before using alternative pharmacological strategies for ADHD, including poor adherence, time action properties of stimulants (and wearing-off of effects), poor tolerability (that prevents the use of higher, more effective doses), excessive focus on or confounding from presence of comorbid non-ADHD symptoms, and tolerance. Finally, we consider the role of non-stimulants and combined pharmacological approaches. While the choice of medication for ADHD is still to a large extent based on a trial-and-error process, there are reasonably accepted data and guidelines to aid in clinical decision-making. It is hoped that advances in precision psychiatry in the years ahead will further guide prescribers to tailor medication choice to the specific characteristics of the patient.
Similar content being viewed by others
References
Cortese S. Pharmacologic treatment of attention deficit-hyperactivity disorder. N Engl J Med. 2020;383(11):1050–6.
Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3:200–11.
Hodgkins P, Shaw M, Coghill D, Hechtman L. Amfetamine and methylphenidate medications for attention-deficit/hyperactivity disorder: complementary treatment options. Eur Child Adolesc Psychiatry. 2012;21(9):477–92.
Ramtvedt BE, Røinås E, Aabech HS, Sundet KS. Clinical gains from including both dextroamphetamine and methylphenidate in stimulant trials. J Child Adolesc Psychopharmacol. 2013;23(9):597–604.
Solanto M, Newcorn J, Vail L, Gilbert S, Ivanov I, Lara R. Stimulant drug response in the predominantly inattentive and combined subtypes of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(6):663–71.
Moukhtarian TR, Cooper RE, Vassos E, Moran P, Asherson P. Effects of stimulants and atomoxetine on emotional lability in adults: a systematic review and meta-analysis. Eur Psychiatry. 2017;44:198–207.
Blader JC, Pliszka SR, Kafantaris V, Foley CA, Carlson GA, Crowell JA, et al. Stepped treatment for attention-deficit/hyperactivity disorder and aggressive behavior: a randomized, controlled trial of adjunctive risperidone, divalproex sodium, or placebo after stimulant medication optimization. J Am Acad Child Adolesc Psychiatry. 2021;60(2):236–51.
Page TF, Pelham WE 3rd, Fabiano GA, Greiner AR, Gnagy EM, Hart KC, et al. Comparative cost analysis of sequential, adaptive, behavioral, pharmacological, and combined treatments for childhood ADHD. J Clin Child Adolesc Psychol. 2016;45(4):416–27.
Pelham WE Jr, Fabiano GA, Waxmonsky JG, Greiner AR, Gnagy EM, Pelham WE 3rd, et al. Treatment sequencing for childhood ADHD: a multiple-randomization study of adaptive medication and behavioral interventions. J Clin Child Adolesc Psychol. 2016;45(4):396–415.
Catalá-López F, Hutton B, Núñez-Beltrán A, Page MJ, Ridao M, Macías Saint-Gerons D, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review with network meta-analyses of randomised trials. PLoS ONE. 2017;12(7):e0180355.
Cortese S, Tomlinson A, Cipriani A. Meta-review: network meta-analyses in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry. 2019;58(2):167–79.
Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727–38.
Coghill D, Seth S. Effective management of attention-deficit/hyperactivity disorder (ADHD) through structured re-assessment: the Dundee ADHD Clinical Care Pathway. Child Adolesc Psychiatry Ment Health. 2015;9:52.
Kovshoff H, Vrijens M, Thompson M, Yardley L, Hodgkins P, Sonuga-Barke EJ, et al. What influences clinicians’ decisions about ADHD medication? Initial data from the Influences on Prescribing for ADHD Questionnaire (IPAQ). Eur Child Adolesc Psychiatry. 2013;22(9):533–42.
Cortese S, Novins DK. Editorial: why JAACAP published an “Inconclusive” trial: optimize, optimize, optimize psychostimulant treatment. J Am Acad Child Adolesc Psychiatry. 2021;60(2):213–5.
Leucht S, Hierl S, Kissling W, Dold M, Davis JM. Putting the efficacy of psychiatric and general medicine medication into perspective: review of meta-analyses. Br J Psychiatry. 2012;200(2):97–106.
Newcorn JH, Sutton VK, Weiss MD, Sumner CR. Clinical responses to atomoxetine in attention-deficit/hyperactivity disorder: the Integrated Data Exploratory Analysis (IDEA) study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):511–8.
Yu C, Garcia-Olivares J, Candler S, Schwabe S, Maletic V. New insights into the mechanism of action of viloxazine: serotonin and norepinephrine modulating properties. J Exp Pharmacol. 2020;12:285–300.
Lloyd KG, Thuret F, Pilc A. Upregulation of gamma-aminobutyric acid (GABA) B binding sites in rat frontal cortex: a common action of repeated administration of different classes of antidepressants and electroshock. J Pharmacol Exp Ther. 1985;235(1):191–9.
Johnson JK, Liranso T, Saylor K, Tulloch G, Adewole T, Schwabe S, et al. A phase II double-blind, placebo-controlled, efficacy and safety study of SPN-812 (Extended-Release Viloxazine) in children with ADHD. J Atten Disord. 2020;24(2):348–58.
Nasser A, Liranso T, Adewole T, Fry N, Hull JT, Chowdhry F, et al. A phase III, randomized, placebo-controlled trial to assess the efficacy and safety of once-daily SPN-812 (Viloxazine Extended-release) in the treatment of attention-deficit/hyperactivity disorder in school-age children. Clin Ther. 2020;42(8):1452–66.
Nasser A, Liranso T, Adewole T, Fry N, Hull JT, Chowdhry F, et al. Once-daily SPN-812 200 and 400 mg in the treatment of ADHD in school-aged children: a phase III Randomized Controlled Trial. Clin Ther. 2021;43(4):684–700.
Faraone SV, Gomeni R, Hull JT, Busse GD, Melyan Z, O’Neal W, et al. Early response to SPN-812 (viloxazine extended-release) can predict efficacy outcome in pediatric subjects with ADHD: a machine learning post-hoc analysis of four randomized clinical trials. Psychiatry Res. 2021;296:113664.
Otasowie J, Castells X, Ehimare UP, Smith CH. Tricyclic antidepressants for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2014;19(9):Cd006997.
Conners CK, Casat CD, Gualtieri CT, Weller E, Reader M, Reiss A, et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1996;35(10):1314–21.
Wilens TE, Haight BR, Horrigan JP, Hudziak JJ, Rosenthal NE, Connor DF, et al. Bupropion XL in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Biol Psychiatry. 2005;57(7):793–801.
Battleday RM, Brem AK. Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: a systematic review. Eur Neuropsychopharmacol. 2016;26(2):391.
Swanson JM, Greenhill LL, Lopez FA, Sedillo A, Earl CQ, Jiang JG, et al. Modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: results of a randomized, double-blind, placebo-controlled, fixed-dose study followed by abrupt discontinuation. J Clin Psychiatry. 2006;67(1):137–47.
Rugino T. A review of modafinil film-coated tablets for attention-deficit/hyperactivity disorder in children and adolescents. Neuropsychiatr Dis Treat. 2007;3(3):293–301.
Arnold VK, Feifel D, Earl CQ, Yang R, Adler LA. A 9-week, randomized, double-blind, placebo-controlled, parallel-group, dose-finding study to evaluate the efficacy and safety of modafinil as treatment for adults with ADHD. J Atten Disord. 2014;18(2):133–44.
Nageye F, Cortese S. Beyond stimulants: a systematic review of randomised controlled trials assessing novel compounds for ADHD. Expert Rev Neurother. 2019;19(7):707–17.
Carlson GA, Dunn D, Kelsey D, Ruff D, Ball S, Ahrbecker L, et al. A pilot study for augmenting atomoxetine with methylphenidate: safety of concomitant therapy in children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health. 2007;1(1):10.
Wilens TE, Bukstein O, Brams M, Cutler AJ, Childress A, Rugino T, et al. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):74-85.e2.
Hazell PL, Stuart JE. A randomized controlled trial of clonidine added to psychostimulant medication for hyperactive and aggressive children. J Am Acad Child Adolesc Psychiatry. 2003;42(8):886–94.
McCracken JT, McGough JJ, Loo SK, Levitt J, Del’Homme M, Cowen J, et al. Combined stimulant and guanfacine administration in attention-deficit/hyperactivity disorder: a controlled, comparative study. J Am Acad Child Adolesc Psychiatry. 2016;55(8):657-66.e1.
Bilder RM, Loo SK, McGough JJ, Whelan F, Hellemann G, Sugar C, et al. Cognitive effects of stimulant, guanfacine, and combined treatment in child and adolescent attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(8):667–73.
Sayer GR, McGough JJ, Levitt J, Cowen J, Sturm A, Castelo E, et al. Acute and long-term cardiovascular effects of stimulant, guanfacine, and combination therapy for attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2016;26(10):882–8.
Kollins SH, Jain R, Brams M, Segal S, Findling RL, Wigal SB, et al. Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with ADHD. Pediatrics. 2011;127(6):e1406–13.
Popper CW. Combining methylphenidate and clonidine: pharmacologic questions and news reports about sudden death. J Child Adolesc Psychopharmacol. 2009;5:3.
Blader JC, Schooler NR, Jensen PS, Pliszka SR, Kafantaris V. Adjunctive divalproex versus placebo for children with ADHD and aggression refractory to stimulant monotherapy. Am J Psychiatry. 2009;166(12):1392–401.
Armenteros JL, Lewis JE, Davalos M. Risperidone augmentation for treatment-resistant aggression in attention-deficit/hyperactivity disorder: a placebo-controlled pilot study. J Am Acad Child Adolesc Psychiatry. 2007;46(5):558–65.
Findling RL, McBurnett K, White C, Youcha S. Guanfacine extended release adjunctive to a psychostimulant in the treatment of comorbid oppositional symptoms in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2014;24(5):245–52.
Wolraich ML, Hagan JF Jr, Allan C, Chan E, Davison D, Earls M, Evans SW, Flinn SK, Froehlich T, Frost J, Holbrook JR, Lehmann CU, Lessin HR, Okechukwu K, Pierce KL, Winner JD, Zurhellen W; Subcommittee on children and adolescents with attention-deficit/hyperactive disorder. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144(4):e20192528. https://doi.org/10.1542/peds.2019-2528.
Canadian ADHD Resource Alliance (CADDRA) Canadian ADHD Practice Guidelines. Fourth Edition, Toronto. 2018.
Association of the Scientific Medical Societies in Germany (AWMF). Interdisciplinary Evidence- and Consensus-based (S3) Guideline “Attention Deficit/Hyperactivity Disorder in Children, Young People and Adults” [in German]. 2018. https://www.awmf.org/uploads/tx_szleitlinien/028-045l_S3_ADHS_2018-06.pdf.
National Institute for Health and Care Excellence. Attention defificit hyperactivity disorder: diagnosis and management. NICE guideline [NG87]. London: NICE; 2018.
Grupo de trabajo de la Guía de Práctica Clínica sobre las Intervenciones Terapéuticas en el Trastorno por Déficit de Atención con Hiperactividad (TDAH). Guía de Práctica Clínica sobre las Intervenciones Terapéuticas en el Trastorno por Déficit de Atención con Hiperactividad (TDAH). Ministerio de Sanidad, Servicios Sociales e Igualdad. Instituto Aragonés de Ciencias de la Salud (IACS); 2017 Guías de Práctica Clínica en el SNS.
Ching C, Eslick GD, Poulton AS. Evaluation of methylphenidate safety and maximum-dose titration rationale in attention-deficit/hyperactivity disorder: a meta-analysis. JAMA Pediatr. 2019;173(7):630–9.
Gualtieri CT, Hicks RE, Patrick K, Schroeder SR, Breese GR. Clinical correlates of methylphenidate blood levels. Ther Drug Monit. 1984;6(4):379–92.
Wang GJ, Volkow ND, Wigal T, Kollins SH, Newcorn JH, Telang F, et al. Long-term stimulant treatment affects brain dopamine transporter level in patients with attention deficit hyperactive disorder. PLoS ONE. 2013;8(5):e63023.
Savill NC, Buitelaar JK, Anand E, Day KA, Treuer T, Upadhyaya HP, et al. The efficacy of atomoxetine for the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a comprehensive review of over a decade of clinical research. CNS Drugs. 2015;29(2):131–51.
Michelson D, Read HA, Ruff DD, Witcher J, Zhang S, McCracken J. CYP2D6 and clinical response to atomoxetine in children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2007;46(2):242–51.
Brown JT, Bishop JR, Sangkuhl K, Nurmi EL, Mueller DJ, Dinh JC, et al. Clinical pharmacogenetics implementation consortium guideline for cytochrome P450 (CYP)2D6 genotype and atomoxetine therapy. Clin Pharmacol Ther. 2019;106(1):94–102.
Ghajar A, Aghajan-Nashtaei F, Afarideh M, Mohammadi MR, Akhondzadeh S. l-Carnosine as adjunctive therapy in children and adolescents with attention-deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled clinical trial. J Child Adolesc Psychopharmacol. 2018;28(5):331–8.
Abbasi SH, Heidari S, Mohammadi MR, Tabrizi M, Ghaleiha A, Akhondzadeh S. Acetyl-l-carnitine as an adjunctive therapy in the treatment of attention-deficit/hyperactivity disorder in children and adolescents: a placebo-controlled trial. Child Psychiatry Hum Dev. 2011;42(3):367–75.
Chuang WC, Yeh CB, Wang SC, Pan PY, Shyu JF, Liu YP, et al. Potential negative effects of dextromethorphan as an add-on therapy to methylphenidate in children with ADHD. Front Psychiatry. 2019;10:437.
Barragán E, Breuer D, Döpfner M. Efficacy and safety of omega-3/6 fatty acids, methylphenidate, and a combined treatment in children with ADHD. J Atten Disord. 2017;21(5):433–41.
Weizman A, Weitz R, Szekely GA, Tyano S, Belmaker RH. Combination of neuroleptic and stimulant treatment in attention deficit disorder with hyperactivity. J Am Acad Child Psychiatry. 1984;23(3):295–8.
Dehbozorghi S, Bagheri S, Moradi K, Shokraee K, Mohammadi MR, Akhondzadeh S. Efficacy and safety of tipepidine as adjunctive therapy in children with attention-deficit/hyperactivity disorder: randomized, double-blind, placebo-controlled clinical trial. Psychiatry Clin Neurosci. 2019;73(11):690–6.
Tashakori A, Khozuey RF. The effect of combination of pramipexole and methylphenidate in the treatment of children with attention deficit hyperactivity disorder. Minerva Psichiatr. 2019;60(3):129–36.
Mohammadpour N, Jazayeri S, Tehrani-Doost M, Djalali M, Hosseini M, Effatpanah M, et al. Effect of vitamin D supplementation as adjunctive therapy to methylphenidate on ADHD symptoms: a randomized, double blind, placebo-controlled trial. Nutr Neurosci. 2018;21(3):202–9.
Noorazar SG, Malek A, Aghaei SM, Yasamineh N, Kalejahi P. The efficacy of zinc augmentation in children with attention deficit hyperactivity disorder under treatment with methylphenidate: a randomized controlled trial. Asian J Psychiatr. 2020;48:101868.
Arnold LE, Disilvestro RA, Bozzolo D, Bozzolo H, Crowl L, Fernandez S, et al. Zinc for attention-deficit/hyperactivity disorder: placebo-controlled double-blind pilot trial alone and combined with amphetamine. J Child Adolesc Psychopharmacol. 2011;21(1):1–19.
Bae S, Park S, Han DH. A mixed herbal extract as an adjunctive therapy for attention deficit hyperactivity disorder: a randomized placebo-controlled trial. Integr Med Res. 2021;10(3):100714.
Connor DF, Barkley RA, Davis HT. A pilot study of methylphenidate, clonidine, or the combination in ADHD comorbid with aggressive oppositional defiant or conduct disorder. Clin Pediatr (Phila). 2000;39(1):15–25.
Coghill D. Pharmacological Approaches in Child and Adolescent Mental Health. In: al. ETe, editor. Mental Health and Illness of Children and Adolescents; 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Not applicable.
Conflict of interest
SC declares honoraria and reimbursement for travel and accommodation expenses for lectures from the following non-profit associations: Association for Child and Adolescent Central Health (ACAMH), Canadian ADHD Alliance Resource (CADDRA), British Association of Pharmacology (BAP), and the Healthcare Convention for educational activity on ADHD. In the past year, JN has been an advisor and/or consultant for Adlon Therapeutics, Arbor, Cingulate Therapeutics, Corium, Eisai, Lundbeck, Medice, Myriad Neuroscience, NLS, OnDosis, Rhodes, Shire/Takeda, and Supernus. He has received research support from the National Institute on Drug Abuse (NIDA), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Adlon, Shire and Supernus. He also has received speaker fees from Shire/Takeda for disease-state presentations and has served as a consultant for the US National Football League. DC has received research support from the National Health and Medical Research Council (NHMRC) Australia, research support and/or honoraria from Shire/Takeda, Medice, and Servier, and royalties from Oxford University Press and Cambridge University Press.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
SC drafted the first version of the manuscript; JN and DC revised it critically. All authors read and approved the final version of the manuscript for submission and publication, and all agree to be accountable for the review.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cortese, S., Newcorn, J.H. & Coghill, D. A Practical, Evidence-informed Approach to Managing Stimulant-Refractory Attention Deficit Hyperactivity Disorder (ADHD). CNS Drugs 35, 1035–1051 (2021). https://doi.org/10.1007/s40263-021-00848-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-021-00848-3