Abstract
Background
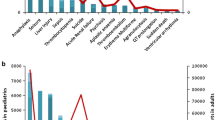
Children are frequently prescribed medication ‘off-label’, meaning there has not been sufficient testing of the medication to determine its safety or effectiveness. The main reason this safety knowledge is lacking is due to ethical restrictions that prevent children from being included in the majority of clinical trials.
Objective
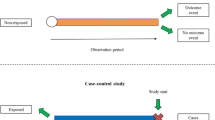
The objective of this paper is to investigate whether an ensemble of simple study designs can be implemented to signal acutely occurring side effects effectively within the paediatric population by using historical longitudinal data. The majority of pharmacovigilance techniques are unsupervised, but this research presents a supervised framework.
Methods
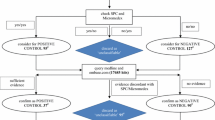
Multiple measures of association are calculated for each drug and medical event pair and these are used as features that are fed into a classifier to determine the likelihood of the drug and medical event pair corresponding to an adverse drug reaction. The classifier is trained using known adverse drug reactions or known non-adverse drug reaction relationships.
Results
The novel ensemble framework obtained a false positive rate of 0.149, a sensitivity of 0.547 and a specificity of 0.851 when implemented on a reference set of drug and medical event pairs. The novel framework consistently outperformed each individual simple study design.
Conclusion
This research shows that it is possible to exploit the mechanism of causality and presents a framework for signalling adverse drug reactions effectively.
Similar content being viewed by others
References
Aagaard L, Christensen A, Hansen EH. Information about adverse drug reactions reported in children: a qualitative review of empirical studies. Br J Clin Pharmacol. 2010;70(4):481–91.
Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, Knoeppel C, Seyberth H, Pandolfini C, Raffaelli MP, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. Br Med J. 2000;320(7227):79–82.
Rose K, van den Anker JN. Guide to paediatric drug development and clinical research. Basel: Karger; 2010.
Bwakura-Dangarembizi M, Musesengwa R, Nathoo KJ, Takaidza P, Mhute T, Vhembo T. Ethical and legal constraints to childrens participation in research in Zimbabwe: experiences from the multicenter pediatric HIV ARROW trial. BioMed Central Med Ethics. 2012;13(1):17.
Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet. 2004;364(9436):803–11.
Lidz CW, Appelbaum PS, Grisso T, Renaud M. Therapeutic misconception and the appreciation of risks in clinical trials. Soc Sci Med. 2004;58(9):1689–97.
Avery A, Anderson C, Bond C, Fortnum H, Gifford A, Hannaford P, Hazell L, Krska J, Lee A, McLernon D, et al. Evaluation of patient reporting of adverse drug reactions to the UK ‘Yellow card scheme’: Literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess. 2011;15(20):1–234.
Aagaard L. Knowledge creation about adverse drug reactions in the paediatric population. Ugeskr Laeger. 2013;175(6):342–45.
Bate A, Evans S. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18(6):427–36.
Ryan PB, Madigan D, Stang PE, Marc-Overhage J, Racoosin JA, Hartzema AG. Empirical assessment of methods for risk identification in healthcare data: results from the experiments of the Observational Medical Outcomes Partnership. Stat Med. 2012;31(30):4401–15.
Reps JM, Garibaldi JM, Aickelin U, Soria D, Gibson J, Hubbard R. Comparison of algorithms that detect drug side effects using electronic healthcare databases. Soft Comput. 2013;17(12):2381–97. doi:10.1007/s00500-013-1097-4.
Jin H, Chen J, He H, Kelman C, McAullay D, O’Keefe CM. Signaling potential adverse drug reactions from administrative health databases. IEEE Trans Knowl Data Eng. 2010;22(6):839–53.
Simpson SE. Self-controlled methods for postmarketing drug safety surveillance in large-scale longitudinal data. Ph.D. thesis, Columbia University; 2011.
Harpaz R, DuMouchel W, Shah NH, Madigan D, Ryan P, Friedman C. Novel data-mining methodologies for adverse drug event discovery and analysis. Clin Pharmacol Ther. 2012;91(6):1010–21.
Zorych I, Madigan D, Ryan P, Bate A. Disproportionality methods for pharmacovigilance in longitudinal observational databases. Stat Methods Med Res. 2013;22(1):39–56.
Norén GN, Hopstadius J, Bate A, Star K, Edwards IR. Temporal pattern discovery in longitudinal electronic patient records. Data Min Knowl Discov. 2010;20(3):361–87.
Greenland S, Neutra R. Control of confounding in the assessment of medical technology. Int J Epidemiol. 1980;9(4):361–67.
Reps JM, Garibaldi JM, Aickelin U, Soria D, Gibson JE, Hubbard RB. A novel prescription drug side effect classifier. IEEE Trans Knowl Data Eng. 2014 (submitted)
Joint Formulary Committee. British National Formulary, 66 ed. London: BMJ Group and Pharmaceutical Press; 2013.
Liaw A, Wiener M. Classification and regression by random forest. R News. 2002;2(3):18–22. http://CRAN.R-project.org/doc/Rnews/
Overhage JM, Ryan PB, Reich CG, Hartzema AG, Stang PE. Validation of a common data model for active safety surveillance research. J Am Med Inf Assoc. 2012;19(1):54–60.
Acknowledgments
This study was funded by the Department of Computer Sciences, University of Nottingham, UK. J.M. Reps, J.M. Garibaldi, U. Aickelin, D. Soria, J.E. Gibson and R.B. Hubbard have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reps, J.M., Garibaldi, J.M., Aickelin, U. et al. Signalling Paediatric Side Effects using an Ensemble of Simple Study Designs. Drug Saf 37, 163–170 (2014). https://doi.org/10.1007/s40264-014-0137-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-014-0137-z