Abstract
Introduction
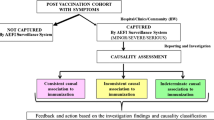
Australia is traditionally an early adopter of vaccines, therefore comprehensive and effective post-licensure vaccine pharmacovigilance is critical to maintain confidence in immunisation, both nationally and internationally. With adverse event following immunisation (AEFI) surveillance the responsibility of Australian jurisdictions, Victoria operates an enhanced passive AEFI surveillance system integrated with clinical services, called ‘SAEFVIC’ (Surveillance of Adverse Events Following Vaccination In the Community).
Objective
The aim of this study was to evaluate Victoria’s current AEFI surveillance system ‘SAEFVIC’ and inform ongoing quality improvement of vaccine pharmacovigilance in Victoria and Australia.
Methods
We conducted a retrospective structured desktop evaluation of AEFI reporting received by SAEFVIC from 2007 to 2014, to evaluate the system according to its stated objectives, i.e. to improve AEFI reporting; provide AEFI signal detection; and to maintain consumer confidence in vaccination.
Results
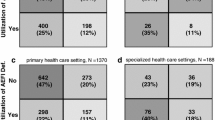
AEFI reporting has tripled since SAEFVIC commenced (incidence risk ratio [IRR] 3.04, 95% confidence interval [CI] 2.35–3.93), raising Victoria to be the lead jurisdiction by AEFI reporting volume and to rank third by population reporting rate nationally. The largest increase was observed in children. Data were utilised to investigate potential signal events and inform vaccine policy. Signal detection required clinical suspicion by surveillance nurses, or prior vaccine-specific concerns. Subsequent vaccination post-AEFI was documented for 56.2% (95% CI 54.1–58.4) of reports, and the proportion of children due or overdue for vaccination was 2.3% higher for those reporting AEFI compared with the general population.
Conclusion
SAEFVIC has improved AEFI surveillance, facilitates signal investigation and validation, and supports consumer confidence in immunisation. Expansion of the system nationally has the potential to improve capacity and capability of vaccine pharmacovigilance, particularly through data consistency and jurisdictional comparability in Australia.


Similar content being viewed by others
Notes
Life-threatening event is an event/reaction in which the client was at risk of death at the time of the event/reaction; it does not refer to an event/reaction that hypothetically might have caused death if it were more severe.
References
Brotherton JM, Gold MS. Monitoring vaccine safety: a critical component of every immunisation program. Med J Aust. 2008;189(5):243–4.
Amarasinghe A, Black S, Bonhoeffer J, Carvalho SMD, Dodoo A, Eskola J, et al. Effective vaccine safety systems in all countries: a challenge for more equitable access to immunization. Vaccine. 2013;31(Suppl 2):B108–14.
Waldman EA, Luhm KR, Monteiro SA, Freitas FR. Surveillance of adverse effects following vaccination and safety of immunization programs. Rev Saude Publica. 2011;45(1):173–84.
Ball R. Perspectives on the future of postmarket vaccine safety surveillance and evaluation. Expert Rev Vaccines. 2014;13(4):455–62.
Chen RT, Shimabukuro TT, Martin DB, Zuber PL, Weibel DM, Sturkenboom M. Enhancing vaccine safety capacity globally: a lifecycle perspective. Vaccine. 2015;33(Suppl 4):D46–54.
World Health Organization. Global vaccine safety blueprint. Geneva: World Health Organization; 2012. WHO/IVB/12.07. http://vaccine-safety-training.org/tl_files/vs/pdf/WHO_IVB_12.07_eng.pdf. Accessed 5 Nov 2016.
Mahajan D, Cook J, Dey A, Macartney K, Menzies RI. Annual report: surveillance of adverse events following immunisation in Australia, 2011. Commun Dis Intell Q Rep. 2012;36(4):E315–32.
Bonhoeffer J, Kohl K, Chen R, Duclos P, Heijbel H, Heininger U, et al. The Brighton Collaboration: addressing the need for standardized case definitions of adverse events following immunization (AEFI). Vaccine. 2002;21(3–4):298–302.
Postila V, Kilpi T. Use of vaccine surveillance data in the evaluation of safety of vaccines. Vaccine. 2004;22(15–16):2076–9.
Letourneau M, Wells G, Walop W, Duclos P. Improving global monitoring of vaccine safety: a survey of national centres participating in the WHO Programme for International Drug Monitoring. Drug Saf. 2008;31(5):389–98.
Bonhoeffer J, Black S, Izurieta H, Zuber P, Sturkenboom M. Current status and future directions of post-marketing vaccine safety monitoring with focus on USA and Europe. Biologicals. 2012;40(5):393–7.
Graham JE, Borda-Rodriguez A, Huzair F, Zinck E. Capacity for a global vaccine safety system: the perspective of national regulatory authorities. Vaccine. 2012;30(33):4953–9.
Lopalco PL, Johansen K, Ciancio B, De Carvalho Gomes H, Kramarz P, Giesecke J. Monitoring and assessing vaccine safety: a European perspective. Expert Rev Vaccines. 2010;9(4):371–80.
Zhou W, Pool V, Iskander JK, English-Bullard R, Ball R, Wise RP, et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)–United States, 1991–2001. MMWR Surveill Summ. 2003;52(1):1–24.
Duclos P. A global perspective on vaccine safety. Vaccine. 2004;22(15–16):2059–63.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96.
McNeil MM, Li R, Pickering S, Real TM, Smith PJ, Pemberton MR. Who is unlikely to report adverse events after vaccinations to the Vaccine Adverse Event Reporting System (VAERS)? Vaccine. 2013;31(24):2673–9.
Monteiro SA, Takano OA, Waldman EA. Evaluation of the Brazilian surveillance system for adverse events following vaccination [in Portuguese]. Rev Bras Epidemiol. 2011;14(3):361–71.
Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J. 2004;23(4):287–94.
Crawford NW, Clothier H, Hodgson K, Selvaraj G, Easton ML, Buttery JP. Active surveillance for adverse events following immunization. Expert Rev Vaccines. 2014;13(2):265–76.
Horvath J. Review of the management of adverse effects associated with Panvax and Fluvax. Canberra: Commonwealth of Australia; 2011. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/53AE51CDFBC05086CA257D7A001A758A/$File/adverse-event-march-2011.pdf. Accessed 5 Oct 2015.
Australian Government Department of Health. Therapeutic Goods Administration Canberra, Australia. http://www.tga.gov.au/. Accessed 22 Feb 2016.
Crawford NW, Cheng A, Andrews N, Charles PG, Clothier HJ, Day T, et al. Guillain–Barré syndrome following influenza A (H1N1/09) immunisation in Victoria Australia: a self-controlled case series. Med J Aust. 2012;197(10):574–8.
Dodd CN, Romio SA, Black S, Vallozi C, Andrews N, Sturkenboom M, et al. International collaboration to assess the risk of Guillain–Barré syndrome following influenza A (H1N1) 2009 monovalent vaccines. Vaccine. 2013;31(40):4448–58.
Wood N, Sheppeard V, Cashman P, Palasanthiran P, Casacelli M, Cannings K, et al. Influenza vaccine safety in children less than 5 years old. The 2010 and 2011 experience in Australia. Pediatr Infect Dis J. 2012;31(2):199–202.
Gold MS, Effler P, Kelly H, Richmond PC, Buttery JP. Febrile convulsions after 2010 seasonal trivalent influenza vaccine: implications for vaccine safety surveillance in Australia. Med J Aust. 2011;193(9):492–3.
Maraskovsky E, Rockman S, Dyson A, Koernig S, Becher D, Morelli AB, et al. Scientific investigations into febrile reactions observed in the paediatric population following vaccination with a 2010 Southern Hemisphere trivalent influenza vaccine. Vaccine. 2012;30(51):7400–6.
Australian Government Department of Health. Seasonal flu vaccine remains suspended for young children without risk factors—advice from the Chief Medical Officer. 2010. http://www.health.gov.au/internet/main/publishing.nsf/Content/mr-yr10-dept-dept010610.htm. Accessed 8 June 2014.
Stokes B. Ministerial review into the public health response into the adverse events to the seasonal influenza vaccine. Government of Western Australia Department of Health, Australia. 2010. https://www.health.wa.gov.au/publications/documents/Stokes_Report.pdf. Accessed 8 June 2015.
Moulton E. Parents of Saba Button who was a victim of flu vaccine debacle receive payout from WA Government Perth. PerthNow Sunday Times. Newspaper article; 2014. http://www.perthnow.com.au/news/western-australia/parents-of-saba-button-who-was-victim-of-flu-vaccine-debacle-receive-payout-from-wa-government/story-fnhocxo3-1226945651845. Accessed 1 Oct 2016.
Mahajan D, Dey A, Cook J, Harvey B, Menzies R, Macartney K. Surveillance of adverse events following immunisation in Australia annual report, 2013. Commun Dis Intell Q Rep. 2015;39(3):E369–86.
Australian Bureau of Statistics. 2011 Census QuickStats. http://www.censusdata.abs.gov.au/census_services/getproduct/census/2011/quickstat/2. Accessed 26 Sep 2013.
Lawrence G, Boyd I, McIntyre P, Isaacs D. Surveillance of adverse events following immunisation: Australia 2002 to 2003. Commun Dis Intell Q Rep. 2004;28(3):324–38.
Clothier HJ, Crawford NW, Kempe A, Buttery JP. Surveillance of adverse events following immunisation: the model of SAEFVIC, Victoria. Commun Dis Intell Q Rep. 2011;35(4):294–8.
German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN, et al. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep. 2001;50(RR-13):1–35.
Lawrence GL, Boyd I, McIntyre PB, Isaacs D. Annual report: surveillance of adverse events following immunisation in Australia, 2004. Commun Dis Intell Q Rep. 2005;29(3):248–62.
Lawrence GL, Aratchige PE, Boyd I, McIntyre PB, Gold MS. Annual report on surveillance of adverse events following immunisation in Australia, 2006. Commun Dis Intell Q Rep. 2007;31(3):269–82.
Lawrence G, Gold MS, Hill R, Deeks S, Glasswell A, McIntyre PB. Annual report: surveillance of adverse events following immunisation in Australia, 2007. Commun Dis Intell Q Rep. 2008;32(4):371–87.
Menzies R, Mahajan D, Gold MS, Roomiani I, McIntyre P, Lawrence G. Annual report: surveillance of adverse events following immunisation in Australia, 2008. Commun Dis Intell Q Rep. 2009;33(4):365–81.
Mahajan D, Roomiani I, Gold MS, Lawrence GL, McIntyre PB, Menzies RI. Annual report: surveillance of adverse events following immunisation in Australia, 2009. Commun Dis Intell. 2010;34(3):259–76.
Mahajan D, Cook J, McIntyre PB, Macartney K, Menzies RI. Annual report: surveillance of adverse events following immunisation in Australia, 2010. Commun Dis Intell Q Rep. 2011;35(4):263–80.
Mahajan D, Dey A, Cook J, Harvey B, Menzies RI, Macartney KM. Surveillance of adverse events following immunisation in Australia, 2012. Commun Dis Intell Q Rep. 2014;38(3):E232–46.
Dey A, Wang H, Quinn HE, Hill R, Macartney K. Surveillance of adverse events following immunisation in Australia annual report, 2014. Commun Dis Intell Q Rep. 2016;40(3):14.
Australian Bureau of Statistics. Australian Demographic Statistics, June 2012. Estimated resident population by single year of age, Victoria 2012. http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Jun2012?OpenDocument. Accessed 23 May 2014.
World Health Organization. Definition and application of terms for vaccine pharmacovigilance. Report of CIOMS/WHO Working Group on Vaccine Pharmacovigilance. Geneva: Council for International Organizations of Medical Sciences; 2012. http://www.who.int/vaccine_safety/initiative/tools/CIOMS_report_WG_vaccine.pdf. Accessed 23 June 2015.
National Health and Medical Research Council. Australian Immunisation Handbook. 10th ed. Canberra: National Capital Press; 2015. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook10-home. Accessed 23 June 2015.
Australian Bureau of Statistics. Australian Statistical Geographic Standard (ASGS): correspondences, July 2011, Postcode 2011 to Local Government Area 2011. Released 27 Jun 2012. http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/1270.0.55.006July%202011?OpenDocument. Accessed 23 May 2014.
Australian Government Department of Human Services. Australian Childhood Immunisation Register for health professionals. http://www.humanservices.gov.au/health-professionals/services/australian-childhood-immunisation-register/. Accessed 17 Sep 2015.
Littlejohn ES, Clothier HJ, Perrett KP, Danchin M. Surveillance of adverse events following the introduction of 13-valent pneumococcal conjugate vaccine in infants, and comparison with adverse events following 7-valent pneumococcal conjugate vaccine, in Victoria, Australia. Hum Vaccin Immunother. 2015;11(7):1828–35.
Buttery JP, Madin S, Crawford NW, Elia S, La Vincente S, Hanieh S, et al. Mass psychogenic response to human papillomavirus vaccination. Med J Aust. 2008;189(5):261–2.
Richards S, Chalkiadis G, Lakshman R, Buttery JP, Crawford NW. Complex regional pain syndrome following immunisation. Arch Dis Child. 2012;97(10):913–5.
Crawford NW, Clothier HJ, Elia S, Lazzaro T, Royle J, Buttery JP. Syncope and seizures following human papillomavirus vaccination: a retrospective case series. Med J Aust. 2011;194(1):16–8.
Cheng DR, Perrett KP, Choo S, Danchin M, Buttery JP, Crawford NW. Pediatric anaphylactic adverse events following immunization in Victoria, Australia from 2007 to 2013. Vaccine. 2015;33(13):1602–7.
Clifford V, Crawford NW, Royle J, Lazzaro T, Danchin M, Perrett KP, et al. Recurrent apnoea post immunisation: informing re-immunisation policy. Vaccine. 2011;29(34):5681–7.
Clothier HJ, Hosking L, Crawford NW, Russell M, Easton ML, Quinn JA, et al. Bacillus Calmette–Guerin (BCG) vaccine adverse events in Victoria, Australia: analysis of reports to an enhanced passive surveillance system. Drug Saf. 2015;38(1):79–86.
Australian Government Department of Health. Immunise Australia Program ACIR—current data. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/acir-curr-data.htm. Accessed 23 Feb 2016.
Clothier HJ, Selvaraj G, Easton ML, Lewis G, Crawford NW, Buttery JP. Consumer reporting of adverse events following immunization. Hum Vaccin Immunother. 2014;10(12):3726–30.
Clothier HJ, Selvaraj G, McMinn A, Lewis G, Crawford NW, Buttery JP. SAEFVIC: surveillance of adverse events following immunisation (AEFI) in Victoria, 2012. Vic Infect Dis Bull. 2014;17(1):2–9.
Australian Government Department of Health Immunise Australia Program. Catch-up: incentives for vaccination providers and general practitioners. 2015. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/news-20150412. Accessed 15 Dec 2015.
Hull BP, Deeks SL, McIntyre PB. The Australian Childhood Immunisation Register: a model for universal immunisation registers? Vaccine. 2009;27(37):5054–60.
Chin LK, Crawford NW, Rowles G, Buttery JP. Australian immunisation registers: established foundations and opportunities for improvement. Euro Surveill. 2012;17(16). http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20148
Skull SA, Nolan TM. Australia needs an expanded immunisation register for further improvements in vaccine delivery and program evaluation. Med J Aust. 2007;187(9):504–5.
Australian Immunisation Register Bill 2015: explanatory memorandum 2015. http://www.aph.gov.au/Parliamentary_Business/Bills_Legislation/Bills_Search_Results/Result?bId=r5526. Accessed 5 Nov 2016.
Department of Health Western Australia. Western Australia Vaccine Safety Surveillance: annual report, 2013. http://www.public.health.wa.gov.au/cproot/5660/2/WAVSS_2013_Annual_report.pdf. Accessed 26 Nov 2015.
Parrella A, Braunack-Mayer A, Gold M, Marshall H, Baghurst P. Healthcare providers’ knowledge, experience and challenges of reporting adverse events following immunisation: a qualitative study. BMC Health Serv Res. 2013;15:313.
Eberth JM, Kline KN, Moskowitz DA, Montealegre JR, Scheurer ME. The role of media and the Internet on vaccine adverse event reporting: a case study of human papillomavirus vaccination. J Adolesc Health. 2014;54(3):289–95.
Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885.
Black S, Eskola J, Siegrist CA, Halsey N, Macdonald N, Law B, et al. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet. 2009;374(9707):2115–22.
Dyda A, MacIntyre CR, Banks E, Kaldor J, Newall AT, McIntyre P, et al. Medicare Benefits Schedule data to monitor influenza immunisation in Australian adults. Public Health Res Pract. 2015;25(4):e2541543.
Parrella A, Gold M, Marshall H, Braunack-Mayer A, Watson M, Baghurst P. Parental views on vaccine safety and future vaccinations of children who experienced an adverse event following routine or seasonal influenza vaccination in 2010. Hum Vacc Immunother. 2014;8(5):662–7.
Casiday RE, Cox AR. Restoring confidence in vaccines by explaining vaccine safety monitoring: is a targeted approach needed? Drug Saf. 2006;29(12):1105–9.
Parrella A, Gold M, Marshall H, Braunack-Mayer A, Baghurst P. Parental perspectives of vaccine safety and experience of adverse events following immunisation. Vaccine. 2013;31(16):2067–74.
Australian Government, Department of Health and Ageing. Review of the management of adverse events associated with Panvax and Fluvax (Horvath Review): outcome of recommendations. http://immunise.health.gov.au/internet/immunise/publishing.nsf/Content/E8085119AE9E28B3CA257D7F000019A5/$File/Horvath-review-outcomes.pdf. Accessed 2 Aug 2013.
Carcione D, Blyth CC, Mak DB, Effler PV. User satisfaction with the Western Australian Vaccine Safety Surveillance (WAVSS) System. Aust NZ J Public Health. 2013;37(3):296.
Acknowledgements
The authors wish to thank the SAEFVIC nursing and administration staff, the paediatricians supporting clinical services, and the SAEFVIC Advisory Board who support the development of SAEFVIC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
SAEFVIC is funded by the Department of Health and Human Services, Melbourne, VIC, Australia. Hazel Clothier is the recipient of an Australian Government Research Training Program Scholarship.
Conflict of interest
Hazel Clothier, Nigel Crawford and Jim Buttery receive salary for their respective employee roles with SAEFVIC. Jim Buttery also serves on data safety monitoring committees for the vaccine manufacturer Seqirus Pty Ltd, for which his employer, Monash Health, receives compensation. Melissa Russell and Heath Kelly have no conflicts of interest that are directly relevant to the content of this study.
Ethical approval
The Human Research Ethics Committee, Royal Childrens Hospital, granted approval for this study (DA017-2013-04).
Rights and permissions
About this article
Cite this article
Clothier, H.J., Crawford, N.W., Russell, M. et al. Evaluation of ‘SAEFVIC’, A Pharmacovigilance Surveillance Scheme for the Spontaneous Reporting of Adverse Events Following Immunisation in Victoria, Australia. Drug Saf 40, 483–495 (2017). https://doi.org/10.1007/s40264-017-0520-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-017-0520-7