Abstract
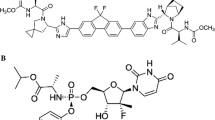
Sofosbuvir (Solvadi™), a nucleotide analogue hepatitis C virus NS5B polymerase inhibitor, is under development with Gilead Sciences for the once-daily, oral treatment of chronic hepatitis C. Oral sofosbuvir has been approved in the US for the treatment of chronic hepatitis C as a component of a combination antiviral regimen. In addition, the European Medicines Agency’s Committee for Medicinal Products for Human Use has recommended the approval of sofosbuvir for the treatment of chronic hepatitis C. This article summarizes the milestones in the development of sofosbuvir leading to this first approval for chronic hepatitis C.
Similar content being viewed by others
References
Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42.
World Health Organization. Hepatitis C: fact sheet no. 164. 2013. http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed 18 Dec 2013.
Stedman CAM. Current prospects for interferon-free treatment of hepatitis C in 2012. J Gastroenterol Hepatol. 2013;28(1):38–45.
Pockros PJ. Interferon-free hepatitis C therapy: how close are we? Drugs. 2012;72:1825–31.
Asselah T. Sofosbuvir-based interferon-free therapy for patients with HCV infection. J Hepatol. 2013;59(6):1342–5.
Gilead Sciences Inc. Solvadi™ (sofosbuvir) tablets, for oral use: US prescribing information. 2013. http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/sovaldi/sovaldi_pi.pdf. Accessed 16 Dec 2013.
US Food and Drug Administration. FDA approves Sovaldi for chronic hepatitis C. 2013. http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/ucm377920.htm. Accessed 16 Dec 2013.
Gilead Sciences Inc. European CHMP adopts positive opinion for Gilead Sciences Sovaldi® for the treatment of chronic hepatitis C infection. 2013. http://www.gilead.com. Accessed 22 Nov 2013.
Gilead Sciences Inc. U.S. Food and Drug Administration approves Gileads Sovaldi™ (sofosbuvir) for the treatment of chronic hepatitis C. 2013. http://www.gilead.com. Accessed 6 Dec 2013.
Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2013;. doi:10.1016/S0140-6736(13)62121-2.
Gilead Sciences Inc. Gilead announces SVR12 rates from three phase 3 studies evaluating a once-daily fixed-dose combination of sofosbuvir and ledipasvir for genotype 1 hepatitis C patients [media release]. 2013. http://www.gilead.com. Accessed 8 Jan 2014.
Guedj J, Pang PS, Lawitz E, et al. Analysis of the kinetics of viral decline during 14 days of administration of sofosbuvir and GS-0938 [abstract no. 1196]. J Hepatol. 2013;58:S486–7.
Hebner C, Lee Y-J, Han B, et al. In vitro pan-genotypic and combination activity of sofosbuvir (GS-7977) in stable replicon cell lines [abstract no. 1875]. Hepatology. 2012;56(4 Suppl):1066A.
Svarovskaia ES, Dvory HS, Hebner C, et al. No resistance detected in four phase 3 clinical studies in HCV genotype 1-6 of sofosbuvir + ribavirin with or without peginterferon [abstract no. 1843]. Hepatology. 2013;58(4 Suppl):1091A–2A.
Svarovskaia ES, Dvory-Sobol H, Gontcharova V, et al. Comprehensive resistance testing in patients who relapsed after treatment with sofosbuvir (GS-7977)-containing regimens in phase 2 studies [abstract no. 753]. Hepatology. 2012;56(4 Suppl):551A.
Kowdley KV, Lawitz E, Crespo I, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381(9883):2100–7.
Lawitz E, Lalezari JP, Hassanein T, et al. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis. 2013;13(5):401–8.
Gane EJ, Stedman CA, Hyland RH, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368(1):34–44.
Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–87.
Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867–77.
Han B, Mo H, Wong KA. In vitro analyses of HCV NS5B S282T mutants in multiple HCV genotypes show low levels of reduced susceptibility to sofosbuvir (GS-7977), no cross resistance to other classes of direct-acting antivirals, and hypersensitivity to ribavirin [abstract no. 1078]. Hepatology. 2012;56(4 Suppl):711A–2A.
Rajyaguru S, Xu S, Hebner C, et al. Sofosbuvir selects the NS5B S282T mutation in vitro in genotype 1-6 replicons and is not cross-resistant to resistance associated variants selected by other classes of antiviral inhibitors [abstract no. 1094]. Hepatology. 2013;58(4 Suppl):739A.
Kirby B, Gordi T, Symonds WT, et al. Population pharmacokinetics of sofosbuvir and its major metabolite (GS-331007) in healthy and HCV infected adult subjects [abstract no. 1106]. Hepatology. 2013;58(4 Suppl):746A–7A.
Mogalian E, German P, Brainard DM, et al. Lack of a clinically significant pharmacokinetic drug-drug interaction between sofosbuvir and GS-5816 in healthy volunteers [abstract no. 465]. Hepatology. 2013;58(4 Suppl):431A.
German P, Mathias A, Pang PS, et al. Lack of a clinically significant pharmacokinetic drug-drug interaction between sofosbuvir (GS-7977) and GS-5885 or GS-9669 in healthy volunteers [abstract no. 1888]. Hepatology. 2012;56(4 Suppl):1072A–3A.
Kirby B, Mathias A, Rossi S, et al. No clinically significant pharmacokinetic drug interactions between sofosbuvir (GS-7977) and HIV antiretrovirals Atripla®, rilpivirine, darunavir/ritonavir, or raltegravir in healthy volunteers [abstract no. 1877]. Hepatology. 2012;56(56 Suppl):1067A.
Mathias A, Cornpropst M, Clemons D, et al. No clinically significant pharmacokinetic drug-drug interactions between sofosbuvir (GS-7977) and the immunosuppressants, cyclosporine A or tacrolimus in healthy volunteers [abstract no. 1869]. Hepatology. 2012;56(4 Suppl):1063A–4A.
German P, Moorehead L, Pang PS, et al. Lack of a clinically important pharmacokinetic interaction between norgestimate/ethinyl estradiol and sofosbuvir (SOF) or ledipasvir (LDV) in HCV-uninfected female subjects [abstract no. 469]. Hepatology. 2013;58(4 Suppl):433A.
Kowdley K, Shiffman M, Sheikh A, et al. Sofosbuvir + ribavirin with or without peginterferon is well-tolerated and associated with high SVR rates: integrated results from 4 phase 3 trials in HCV genotype 1-6 [abstract no. 412]. Am J Gastroenterol. 2013;108(Suppl 1):S123.
Lawitz E, Poordad F, Brainard DM, et al. Sofosbuvir in combination with PegIFN and ribavirin for 12 weeks provides high SVR rates in HCV-infected genotype 2 or 3 treatment-experienced patients with and without compensated cirrhosis: results from the LONESTAR-2 study [abstract no. LB-4]. Hepatology. 2013;58(6 Suppl):1380A.
Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir + ribavirin for 12 or 24 weeks for patients with HCV genotype 2 or 3: the VALENCE trial [abstract no. 1085]. Hepatology. 2013;58(4 Suppl):733A–4A.
Ruane PJ, Ain D, Riad J, et al. Sofosbuvir plus ribavirin in the treatment of chronic HCV genotype 4 infection in patients of Egyptian ancestry [abstract no. 1090]. Hepatology. 2013;58(4 Suppl):736A.
Sulkowski MS, Rodriguez-Torres M, Lalezari JP, et al. All-oral therapy with sofosbuvir plus ribavirin for the treatment of HCV genotype 1, 2, and 3 infection in patients co-infected with HIV (PHOTON-1) [abstract no. 212]. Hepatology. 2013;58(4 Suppl):313A–4A.
Curry MP, Forns X, Chung RT, et al. Pretransplant sofosbuvir and ribavirin to prevent recurrence of HCV infection after liver transplantation [abstract no. 213]. Hepatology. 2013;58(4 Suppl):314A–5A.
Charlton MR, Gane EJ, Manns MP, et al. Sofosbuvir and ribavirin for the treatment of established recurrent hepatitis C infection after liver transplantation: preliminary results of a prospective, multicenter study [abstract no. LB-2]. Hepatology. 2013;58(6 Suppl):1378A.
Jacobsen IM, Ghalib RH, Rodriguez-Torres M, et al. SVR results of a once-daily regimen of simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in cirrhotic and non-cirrhotic HCV genotype 1 treatment-naive and prior null responder patients: the COSMOS study [abstract no. LB-3]. Hepatology. 2013;58(6 Suppl):1379A.
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. High rate of sustained virologic response with the all- oral combination of daclatasvir (NS5A inhibitor) plus sofosbuvir (nucleotide NS5B inhibitor), with or without ribavirin, in treatment-naive patients chronically infected with HCV genotype 1, 2, or 3 [abstract no. LB-2]. Hepatology. 2012;56(6):1516A–7A.
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Sustained virologic response with daclatasvir plus sofosbuvir +/− ribavirin (RBV) in chronic HCV genotype (GT) 1-infected patients who previously failed telaprevir (TVR) or boceprevir (BOC) [abstract]. J Gastroenterol Hepatol. 2013;28(Suppl 2):155.
Osinusi A, Meissner EG, Lee Y-J, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310(8):804–11.
Gilead Sciences Inc. Gilead announces phase 2 results for sofosbuvir-based regimens in hepatitis C patients before and after liver transplantation [media release]. 2013. http://www.gilead.com. Accessed 8 Jan 2014.
Gilead Sciences. Sofosbuvir + RBV for 16 or 24 weeks and sofosbuvir + PEG + RBV for 12 weeks in subjects with genotype 2 or 3 chronic HCV infection [ClinicalTrials.gov identifier NCT01962441] US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01962441. Accessed 17 Dec 2013.
Gilead Sciences. Open-label study of sofusbuvir + ribavirin with or without peginterferon alfa-2a in subjects with chronic HCV infection who participated in prior Gilead HCV studies [ClinicalTrials.gov identifier NCT01625338] US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01625338. Accessed 17 Dec 2013.
Gilead Sciences. Safety and efficacy study of sofosbuvir plus ribavirin in treatment-naive adults with genotype 1 and 3 chronic HCV infection [ClinicalTrials.gov identifier NCT01896193] US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01896193. Accessed 17 Dec 2013.
Gilead Sciences. Phase 3 study of sofosbuvir and ribavirin [ClinicalTrials.gov identifier NCT01910636] US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01910636. Accessed 17 Dec 2013.
Gilead Sciences. A phase 3, open-label study to investigate the efficacy and safety of sofosbuvir plus ribavirin in chronic genotype 1, 2, 3 and 4 hepatitis C virus (HCV) and human immunodeficiency virus (HIV) co-infected subjects [ClinicalTrials.gov identifier NCT01783678] US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01783678. Accessed 17 Dec 2013.
Gilead Sciences. Sofosbuvir plus ribavirin administered for either 12 or 24 weeks in treatment-naïve and treatment-experienced Egyptian adults with chronic genotype 4 hepatitis C virus (HCV) infection [ClinicalTrials.gov identifier NCT01713283] US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01713283. Accessed 17 Dec 2013.
Gilead Sciences. Sofosbuvir containing regimens for the treatment of chronic HCV infection in subjects with chronic genotype 1, 2, 3, or 6 HCV infection [ClinicalTrials.gov identifier NCT01826981] US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01826981. Accessed 17 Dec 2013.
Gilead Sciences. GS-7977 and ribavirin in patients with chronic HCV with cirrhosis and portal hypertension with or without liver decompensation [ClinicalTrials.gov identifier NCT01687257] US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01687257. Accessed 17 Dec 2013.
Gilead Sciences. Sofosbuvir plus ribavirin in subjects with HCV infection and renal insufficiency [ClinicalTrials.gov identifier NCT01958281] US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01958281. Accessed 17 Dec 2013.
Gilead Sciences. Safety and efficacy of sofosbuvir plus GS-5816 with or without ribavirin in treatment-naive subjects with chronic HCV infection [ClinicalTrials.gov identifier NCT01858766] US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01858766. Accessed 18 Dec 2013.
Gilead Sciences. Safety and efficacy of sofosbuvir plus GS-5816 with or without ribavirin in treatment-experienced subjects with chronic HCV infection [ClinicalTrials.gov identifier NCT01909804] US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01909804. Accessed 17 Dec 2013.
Gilead Sciences. Expanded access program of sofosbuvir with ribavirin and with or without pegylated interferon in aggressive post-transplant hepatitis C [ClinicalTrials.gov identifier NCT01779518] US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01779518. Accessed 17 Dec 2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Keating, G.M., Vaidya, A. Sofosbuvir: First Global Approval. Drugs 74, 273–282 (2014). https://doi.org/10.1007/s40265-014-0179-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0179-7