Abstract
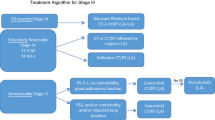
Rearrangements of the anaplastic lymphoma kinase (ALK) gene originally discovered nearly 20 years ago in the context of anaplastic large cell lymphoma were identified as oncogenic drivers in a subset of non-small cell lung cancers (NSCLCs) in 2007. These ALK gene rearrangements are present in 3–5 % of NSCLC patients, typically younger, never or light smokers with adenocarcinomas. Crizotinib is a first-in-class ALK tyrosine kinase inhibitor with significant activity in ALK-positive NSCLC that received accelerated US Food and Drug Administration approval for treatment of ALK-positive NSCLC in 2011, just 4 years after identification of ALK rearrangements in this setting. Subsequently, two phase III trials have shown crizotinib to have a tolerable toxicity profile and to be superior to standard chemotherapy for the first- or second-line treatment of advanced ALK-positive lung cancer and numerous countries have approved its use. Despite initial responses, acquired resistance to crizotinib invariably leads to disease progression. Mechanisms of resistance have been described to include ALK tyrosine kinase mutations, activation of bypass signalling pathways and pharmacokinetic failure of crizotinib. Several next-generation ALK inhibitors, including ceritinib and alectinib, are in clinical development and show efficacy in both the crizotinib naïve and crizotinib refractory settings. Ongoing clinical trials will identify the optimal strategy to incorporate these novel agents in the treatment of patients with ALK-positive NSCLC.



Similar content being viewed by others
References
Solomon B, Varella-Garcia M, Camidge DR. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J Thorac Oncol. 2009;4(12):1450–4. doi:10.1097/JTO.0b013e3181c4dedb.
Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. doi:10.1056/NEJMoa1214886.
Solomon BJ, Mok T, Kim D-W, Wu Y-L, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77.
Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263(5151):1281–4.
Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13(10):685–700. doi:10.1038/nrc3580.
Shaw AT, Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res. 2011;17(8):2081–6. doi:10.1158/1078-0432.CCR-10-1591.
Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14(4):439–49.
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6. doi:10.1038/nature05945.
Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–203. doi:10.1016/j.cell.2007.11.025.
Ou S-HI, Bartlett CH, Mino-Kenudson M, Cui J, Iafrate AJ. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist. 2012;17(11):1351–75.
Soda M, Takada S, Takeuchi K, Choi YL, Enomoto M, Ueno T, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci USA. 2008;105(50):19893–7. doi:10.1073/pnas.0805381105.
Chen Z, Sasaki T, Tan X, Carretero J, Shimamura T, Li D, et al. Inhibition of ALK, PI3K/MEK, and HSP90 in murine lung adenocarcinoma induced by EML4-ALK fusion oncogene. Cancer Res. 2010;70(23):9827–36. doi:10.1158/0008-5472.CAN-10-1671.
McDermott U, Iafrate AJ, Gray NS, Shioda T, Classon M, Maheswaran S, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008;68(9):3389–95. doi:10.1158/0008-5472.CAN-07-6186.
Heuckmann JM, Balke-Want H, Malchers F, Peifer M, Sos ML, Koker M, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res. 2012;18(17):4682–90. doi:10.1158/1078-0432.CCR-11-3260.
Mano H. ALKoma: a cancer subtype with a shared target. Cancer Discov. 2012;2(6):495–502. doi:10.1158/2159-8290.CD-12-0009.
van Gaal JC, Flucke UE, Roeffen MH, de Bont ES, Sleijfer S, Mavinkurve-Groothuis AM, et al. Anaplastic lymphoma kinase aberrations in rhabdomyosarcoma: clinical and prognostic implications. J Clin Oncol. 2012;30(3):308–15. doi:10.1200/JCO.2011.37.8588.
Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363(18):1734–9. doi:10.1056/NEJMoa1007478.
Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4(120):120ra17. doi:10.1126/scitranslmed.3003316.
Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–82.
Yoshida A, Tsuta K, Nakamura H, Kohno T, Takahashi F, Asamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol. 2011;35(8):1226–34. doi:10.1097/PAS.0b013e3182233e06.
Gainor JF, Varghese AM, Ou SH, Kabraji S, Awad MM, Katayama R, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19(15):4273–81. doi:10.1158/1078-0432.CCR-13-0318.
Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–53. doi:10.1200/JCO.2009.22.6993.
Atherly AJ, Camidge DR. The cost-effectiveness of screening lung cancer patients for targeted drug sensitivity markers. Br J Cancer. 2012;106(6):1100–6. doi:10.1038/bjc.2012.60.
Doebele RC, Lu X, Sumey C, Maxson DA, Weickhardt AJ, Oton AB, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer. 2012;118(18):4502–11. doi:10.1002/cncr.27409.
Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. doi:10.1200/JCO.2014.59.0539 (Epub 2015 Jan 15).
Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823–59. doi:10.1097/JTO.0b013e318290868f.
Tsao M, Hirsch F, Yatabe Y. IASLC atlas of ALK testing in lung cancer. Aurora: Int Soc Study Lung Cancer; 2013.
Shaw AT, Solomon B, Kenudson MM. Crizotinib and testing for ALK. J Natl Compr Canc Netw. 2011;9(12):1335–41.
Mino-Kenudson M, Chirieac LR, Law K, Hornick JL, Lindeman N, Mark EJ, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res. 2010;16(5):1561–71. doi:10.1158/1078-0432.CCR-09-2845.
Selinger CI, Rogers TM, Russell PA, O’Toole S, Yip P, Wright GM, et al. Testing for ALK rearrangement in lung adenocarcinoma: a multicenter comparison of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol. 2013;26(12):1545–53. doi:10.1038/modpathol.2013.87.
Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–9. doi:10.1016/S1470-2045(12)70344-3.
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703. doi:10.1056/NEJMoa1006448.
Kim DK, Ahn MJ, Yang P, Liu X, De Pas T, Crino L et al. Updated results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC). Ann Oncol. 2012;23(9):402.
Kazandjian D, Blumenthal GM, Chen HY, He K, Patel M, Justice R, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19(10):e5–11. doi:10.1634/theoncologist.2014-0241.
Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12(11):1004–12. doi:10.1016/S1470-2045(11)70232-7.
Clinical Lung Cancer Genome Project (CLCGP); Network Genomic Medicine (NGM). A genomics-based classification of human lung tumors. Sci Transl Med. 2013;5(209):209ra153.
Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi:10.1001/jama.2014.3741.
Shaw AT, Ou S-HI, Bang Y-J, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non–small-cell lung cancer. N Engl J Med. 2014;371:1963–71. doi:10.1056/NEJMoa1406766.
Mazières J, Zalcman G, Crino L, Biondani P, Barlesi F, Filleron T. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33(9):992–9. doi:10.1200/JCO.2014.58.3302.
Camidge D, Ou S, Shapiro G, Otterson G, Villaruz L, Villalona-Calero M et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC) [abstract no. 8001]. J Clin Oncol. 2014;32(s15).
Solomon B. Refining the Toxicity Profile of Crizotinib. J Thorac Oncol. 2014;9(11):1596–7.
Camidge DR, Doebele RC. Treating ALK-positive lung cancer—early successes and future challenges. Nat Rev Clin Oncol. 2012;9(5):268–77. doi:10.1038/nrclinonc.2012.43.
Lovly CM, Shaw AT. Molecular pathways: resistance to kinase inhibitors and implications for therapeutic strategies. Clin Cancer Res. 2014;20(9):2249–56.
Crystal AS, Shaw AT, Sequist LV, Friboulet L, Niederst MJ, Lockerman EL, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346(6216):1480–6.
Costa DB, Kobayashi S, Pandya SS, Yeo W-L, Shen Z, Tan W, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–5.
Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA Jr, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7(12):1807–14. doi:10.1097/JTO.0b013e3182745948.
Galkin AV, Melnick JS, Kim S, Hood TL, Li N, Li L, et al. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proc Natl Acad Sci USA. 2007;104(1):270–5. doi:10.1073/pnas.0609412103.
Marsilje TH, Pei W, Chen B, Lu W, Uno T, Jin Y, et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-n2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-n4-(2-(isopropylsulf onyl)phenyl)pyrimidine-2,4-diamine (ldk378) currently in phase 1 and phase 2 clinical trials. J Med Chem. 2013;56(14):5675–90. doi:10.1021/jm400402q.
Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–97. doi:10.1056/NEJMoa1311107.
Felip E, Kim D, Mehra R, Tan DSW, Chow LQ, Camidge DR et al. Efficacy and safety of ceritinib in patients (pts) with advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCL) [abstract no. 1295P]. Ann Oncol. 2014;25 (suppl_4):iv426–iv70.
Khozin S, Blumenthal GM, Zhang L, Tang S, Brower M, Fox E et al. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res. 2015;22(11):1–4. doi:10.1158/1078-0432.CCR-14-3157.
Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19(5):679–90. doi:10.1016/j.ccr.2011.04.004.
Kinoshita K, Asoh K, Furuichi N, Ito T, Kawada H, Hara S, et al. Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802). Bioorg Med Chem. 2012;20(3):1271–80. doi:10.1016/j.bmc.2011.12.021.
Kodama T, Tsukaguchi T, Satoh Y, Yoshida M, Watanabe Y, Kondoh O, et al. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol Cancer Ther. 2014;13(12):2910–8. doi:10.1158/1535-7163.MCT-14-0274.
Seto T, Kiura K, Nishio M, Nakagawa K, Maemondo M, Inoue A, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14(7):590–8. doi:10.1016/S1470-2045(13)70142-6.
Gadgeel SM, Gandhi L, Riely GJ, Chiappori AA, West HL, Azada MC, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15(10):1119–28. doi:10.1016/S1470-2045(14)70362-6.
Kodama T, Hasegawa M, Takanashi K, Sakurai Y, Kondoh O, Sakamoto H. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74(5):1023–8. doi:10.1007/s00280-014-2578-6.
Nanjo S, Nakagawa T, Takeuchi S, Kita K, Fukuda K, Nakada M, et al. In vivo imaging models of bone and brain metastases and pleural carcinomatosis with a novel human EML4-ALK lung cancer cell line. Cancer Sci. 2015;106(3):244–52. doi:10.1111/cas.12600.
Gainor JF, Sherman CA, Willoughby K, Logan J, Kennedy E, Brastianos PK, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol. 2015;10(2):232–6. doi:10.1097/JTO.0000000000000455.
Zhang S, Wang F, Keats J, Ning Y, Wardwell SD, Moran L, et al. AP26113, a potent ALK inhibitor, overcomes mutations in EML4-ALK that confer resistance to PF-02341066 (PF1066) [abstract no. LB-298]. AACR 101st annual meeting 2010; 15–17 Apr 2010; Washington, DC.
Rivera VM, Wang F, Anjum R, Zhang S, Squillace R, Keats J, et al. AP26113 is a dual ALK/EGFR inhibitor: characterization against EGFR T790M in cell and mouse models of NSCLC [abstract no. 1794]. AACR 103rd annual meeting; 31 Mar–4 Apr 2012; Chicago.
Gettinger S, Bazhenova L, Salgia R, Langer C, Gold K, Rosell R et al. ALK inhibitor AP26113 in patients with advanced malignancies, including ALK+ non-small cell lung cancer (NSCLC): updated efficacy and safety data [abstract no. 1292P]. Ann Oncol. 2014;25 (suppl_4):iv426–iv70.
Lovly CM, Heuckmann JM, de Stanchina E, Chen H, Thomas RK, Liang C, et al. Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res. 2011;71(14):4920–31. doi:10.1158/0008-5472.CAN-10-3879.
Horn L, Blumenscheine G, Wakelee HA, Arkenau TH, Dukart G, Harrow K, et al. A phase 1 trial of X-396, a novel ALK inhibitor, in patients with advanced solid tumors. Int J Radiat Oncol. 2014;90(5):S52–3.
Wilcoxen KM, Brake RL, Saffran D, Teffera Y, Choquette D, Whittington D et al.. Characterization of a novel series of potent, selective inhibitors of wild type and mutant/fusion anaplastic lymphoma kinase [abstract no. 1795]. AACR 103rd annual meeting; 31 Mar–4 Apr 2012; Chicago.
Sachdev J, Arkenau HT, Infante JR, Mita MM, Anthony SP, Natale RB, et al. Phase (Ph) 1/2a study of TSR-011, a potent inhibitor of ALK and TRK, in advanced solid tumors including crizotinib-resistant ALK positive non-small cell lung cancer [abstract no. 8063]. Eur J Cancer. 2014;50(6):165.
De Braud FGM, L. Pilla L, Niger M, Damian S, Bardazza B, Martinetti A et al. Phase 1 open label, dose escalation study of RXDX-101, an oral Pan-Trk, ROS1, and ALK inhibitor, in patients with advanced solid tumors with relevant molecular alterations [abstract no. 2502]. ESMO; 2014: Ann Oncol. 2014;25(suppl_4):iv146–iv164.
Yamazaki S, Lam JL, Zou HY, Wang H, Smeal T, Vicini P. Translational pharmacokinetic-pharmacodynamic modeling for an orally available novel inhibitor of anaplastic lymphoma kinase and c-Ros oncogene 1. J Pharmacol Exp Ther. 2014;351(1):67–76. doi:10.1124/jpet.114.217141.
Johnson TW, Richardson PF, Bailey S, Brooun A, Burke BJ, Collins MR, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h][2, 5, 11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57(11):4720–44. doi:10.1021/jm500261q.
Bonvini P, Gastaldi T, Falini B, Rosolen A. Nucleophosmin-anaplastic lymphoma kinase (NPM-ALK), a novel Hsp90-client tyrosine kinase: down-regulation of NPM-ALK expression and tyrosine phosphorylation in ALK(+) CD30(+) lymphoma cells by the Hsp90 antagonist 17-allylamino,17-demethoxygeldanamycin. Cancer Res. 2002;62(5):1559–66.
Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci USA. 2011;108(18):7535–40. doi:10.1073/pnas.1019559108.
Normant E, Paez G, West KA, Lim AR, Slocum KL, Tunkey C, et al. The Hsp90 inhibitor IPI-504 rapidly lowers EML4-ALK levels and induces tumor regression in ALK-driven NSCLC models. Oncogene. 2011;30(22):2581–6. doi:10.1038/onc.2010.625.
Sang J, Acquaviva J, Friedland JC, Smith DL, Sequeira M, Zhang C, et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov. 2013;3(4):430–43. doi:10.1158/2159-8290.CD-12-0440.
Sequist LV, Gettinger S, Senzer NN, Martins RG, Janne PA, Lilenbaum R, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol. 2010;28(33):4953–60. doi:10.1200/JCO.2010.30.8338.
Socinski MA, Goldman J, El-Hariry I, Koczywas M, Vukovic V, Horn L, et al. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clin Cancer Res. 2013;19(11):3068–77. doi:10.1158/1078-0432.CCR-12-3381.
Felip E, Carcereny E, Barlesi F, Gandhi L, Sequist LV, Kim S-W, et al. Phase II activity of the HSP90 inhibitor AUY922 in patients with ALK-rearranged (ALK+) or EGFR-mutated advanced non-small cell lung cancer (NSCLC) [abstract no. 4380]. Ann Oncol. 2012;23(9):ix152.
Compliance with ethical standards
Funding: No sources of funding were used to assist with the preparation of this review.
Conflicts of interest: Dr. Cameron has no conflict of interest to declare. Dr. Solomon has served on advisory boards for Pfizer, Roche and Novartis.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the topical collection on Lung Cancer.
Rights and permissions
About this article
Cite this article
Cameron, L., Solomon, B. Treatment of ALK-Rearranged Non-Small Cell Lung Cancer: Recent Progress and Future Directions. Drugs 75, 1059–1070 (2015). https://doi.org/10.1007/s40265-015-0415-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0415-9