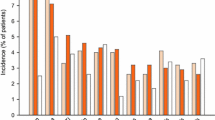
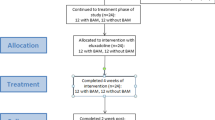
Abstract
Eluxadoline (Viberzi™), developed by Actavis (now Allergan), is an orally administered, first-in-class compound that functions as both a µ-opioid receptor agonist and a δ-opioid receptor antagonist, and is indicated for the treatment of diarrhoea-predominant irritable bowel syndrome (IBS-D) in adults. The agent was originally developed by Janssen Pharmaceutica. Eluxadoline has been approved in the US, and submissions to other global authorities are being contemplated or planned. This article summarizes the milestones in the development of eluxadoline leading to this first approval for IBS-D.
Similar content being viewed by others
References
World Gastroenterology Organisation. Irritable bowel syndrome: a global perspective. 2009. http://www.worldgastroenterology.org. Accessed 17 June 2015.
Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl. 1):S2–26.
NIHR Horizon Scanning Centre. Eluxadoline for irritable bowel syndrome (diarrhoea-predominant)—first line: NIHR Horizon Scanning Centre, University of Birmingham, 2014. Contract No.: 6758.
Barboza JL, Talley NJ, Moshiree B. Current and emerging pharmacotherapeutic options for irritable bowel syndrome. Drugs. 2014;74(16):1849–70.
Actavis. Viberzi™ (eluxadoline tablets): US prescribing information. 2015. http://www.actavis.com. Accessed 15 June 2015.
U.S. Food and Drug Administration. FDA approves two therapies to treat IBS-D [media release]. 27 May 2015. http://www.fda.gov/.
Actavis. Actavis receives FDA approval for VIBERZI (eluxadoline) for the treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults [media release]. 27 May 2015. http://www.actavis.com.
PPD Inc. PPD and Janssen Pharmaceutica collaborate to develop compounds for treatment of irritable bowel syndrome and bacterial infections [media release]. 17 Nov 2009. http://www.ppdi.com.
PPD Inc. PPD and Furiex announce completion of spin-off [media release]. 15 Jun 2010. http://www.ppdi.com.
Furiex Pharmaceuticals Inc. Furiex acquires full exclusive license to MuDelta for the treatment of diarrhea-predominant IBS and reports positive end of phase II FDA meeting [media release]. 1 Nov 2011. http://www.furiex.com.
Forest Pharmaceuticals. Forest laboratories to acquire Furiex Pharmaceuticals for $1.1 billion in cash to build on a leading position in gastroenterology (GI) [media release]. 28 Apr 2014. http://www.frx.com.
Actavis. Actavis plc is now Allergan plc [media release]. 15 June 2015. http://www.actavis.com/.
Davenport JM, Covington P, Bonifacio L, et al. Effect of uptake transporters OAT3 and OATP1B1 and efflux transporter MRP2 on the pharmacokinetics of eluxadoline. J Clin Pharmacol. 2015;55(5):534–42.
Wade PR, Palmer JM, McKenney S, et al. Modulation of gastrointestinal function by MuDelta, a mixed micro opioid receptor agonist/ micro opioid receptor antagonist. Br J Pharmacol. 2012;167(5):1111–25.
Dove LS, Lembo A, Randall CW, et al. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology. 2013;145(2):329–38.e1.
Lacy BE, Dove S, Andrae D, et al. Robustness of eluxadoline for the treatment of irritable bowel syndrome with diarrhea: results from phase 3 composite endpoint assessments [abstract no. Su1378]. Gastroenterology. 2015;148(4 Supplement):S491.
Chey WD, Dove S, Andrae D, et al. Eluxadoline demonstrates sustained efficacy for the treatment of diarrhea-predominant irritable bowel syndrome in phase 3 clinical trials [abstract no. 316]. Gastroenterology. 2015;148(4 Supplement 1):S70-S1.
Zuckerman M, Dove S, Andrae D, et al. Consistency in efficacy outcomes of eluxadoline-treated patients with irritable bowel syndrome with diarrhea using recent and traditional endpoints [abstract no. Su1380]. Gastroenterology. 2015;148(4 Supplement):S492.
Andrae D, Buono JL, Covington PS. Effect of eluxadoline on health-related quality of life in adults with irritable bowel syndrome with diarrhea: Results from two randomized, double-blind, placebo-controlled phase 3 trials [abstract no. Su1385]. Gastroenterology. 2015;148(4 Supplement 1):S494.
Lembo A, Andrae D, Dove S, et al. Urgency as a measure of treatment effect due to eluxadoline [abstract no. 1834]. Am J Gastroenterol. 2014;109(Supplement 2):S542–3.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. K. P. Garnock-Jones is a salaried employee of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Garnock-Jones, K.P. Eluxadoline: First Global Approval. Drugs 75, 1305–1310 (2015). https://doi.org/10.1007/s40265-015-0436-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0436-4