Abstract
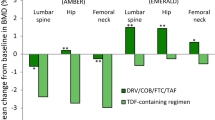
Tenofovir alafenamide (tenofovir AF) is a novel oral prodrug of the nucleos(t)ide reverse transcriptase inhibitor (NRTI) tenofovir that has several pharmacological advantages over tenofovir disoproxil fumarate (tenofovir DF), including increased plasma stability and reduced tenofovir systemic exposure. Tenofovir AF has been coformulated with elvitegravir, cobicistat and emtricitabine as a once-daily, single-tablet regimen (elvitegravir/cobicistat/emtricitabine/tenofovir AF; Genvoya®) for the treatment of adults and adolescents with HIV-1 infection. With regard to establishing and/or maintaining virological suppression over 48 weeks in randomized, phase III trials, elvitegravir/cobicistat/emtricitabine/tenofovir AF was noninferior to elvitegravir/cobicistat/emtricitabine/tenofovir DF in antiretroviral therapy (ART)-naive adults, and statistically superior (subsequent to established noninferiority) to ongoing treatment with tenofovir DF-containing regimens in ART-experienced adults with virological suppression. In single-arm, phase III trials, elvitegravir/cobicistat/emtricitabine/tenofovir AF also provided high rates of virological suppression among ART-naive adolescents and ART-experienced adults with stable renal impairment. In general, elvitegravir/cobicistat/emtricitabine/tenofovir AF was well tolerated and associated with more favourable renal and bone parameters, but a less favourable lipid profile, than tenofovir DF-containing regimens. Thus, elvitegravir/cobicistat/emtricitabine/tenofovir AF is an alternative single-tablet regimen for adults and adolescents with HIV-1 infection, particularly those with an estimated creatinine clearance of ≥30 to <50 mL/min or an increased risk of tenofovir DF-related bone toxicity.

Similar content being viewed by others
References
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. 2016. http://aidsinfo.nih.gov. Accessed 27 Apr 2016.
Günthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312(4):410–25.
EACS: European AIDS Clinical Society. Guidelines version 8.0. 2015. http://eacsociety.org. Accessed 27 Apr 2016.
British HIV Association. BHIVA guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy. 2015. http://www.bhiva.org. Accessed 27 Apr 2016.
Lyseng-Williamson KA, Reynolds NA, Plosker GL. Tenofovir disoproxil fumarate: a review of its use in the management of HIV infection. Drugs. 2005;65(3):413–32.
Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. 2013;207(9):1359–69.
Gupta SK, Anderson AM, Ebrahimi R, et al. Fanconi syndrome accompanied by renal function decline with tenofovir disoproxil fumarate: a prospective, case-control study of predictors and resolution in HIV-infected patients. PLoS One. 2014;9(3):e92717.
McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203(12):1791–801.
Lee WA, He GX, Eisenberg E, et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother. 2005;49(5):1898–906.
Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006;50(10):3297–304.
Rodríguez-Nóvoa S, Labarga P, D’Avolio A, et al. Impairment in kidney tubular function in patients receiving tenofovir is associated with higher tenofovir plasma concentrations. AIDS. 2010;24(7):1064–6.
Callebaut C, Stepan G, Tian Y, et al. In vitro virology profile of tenofovir alafenamide, a novel oral prodrug of tenofovir with improved antiviral activity compared to that of tenofovir disoproxil fumarate. Antimicrob Agents Chemother. 2015;59(10):5909–16.
Bam RA, Yant SR, Cihlar T. Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther. 2014;19(7):687–92.
Gilead Sciences Inc. Genvoya® (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets, for oral use: US prescribing information. 2015. http://www.fda.gov. Accessed 27 Apr 2016.
Gilead Sciences International Ltd. Genvoya 150 mg/150 mg/200 mg/10 mg film-coated tablets: EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 27 Apr 2016.
Deeks ED. Elvitegravir: a review of its use in adults with HIV-1 infection. Drugs. 2014;74(6):687–97.
Deeks ED. Cobicistat: a review of its use as a pharmacokinetic enhancer of atazanavir and darunavir in patients with HIV-1 infection. Drugs. 2014;74(2):195–206.
Frampton JE, Perry CM. Emtricitabine: a review of its use in the management of HIV infection. Drugs. 2005;65(10):1427–48.
Bam RA, Birkus G, Babusis D, et al. Metabolism and antiretroviral activity of tenofovir alafenamide in CD4+ T-cells and macrophages from demographically diverse donors. Antivir Ther. 2014;19(7):669–77.
Margot NA, Johnson A, Miller MD, et al. Characterization of HIV-1 resistance to tenofovir alafenamide in vitro. Antimicrob Agents Chemother. 2015;59(10):5917–24.
Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63(4):449–55.
Markowitz M, Zolopa A, Squires K, et al. Phase I/II study of the pharmacokinetics, safety and antiretroviral activity of tenofovir alafenamide, a new prodrug of the HIV reverse transcriptase inhibitor tenofovir, in HIV-infected adults. J Antimicrob Chemother. 2014;69(5):1362–9.
Sax PE, Zolopa A, Brar I, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2014;67(1):52–8.
Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–15.
Kitrinos KM, Fordyce MW, McCallister S, et al. Integrated analysis of emergent drug resistance through 48 weeks from clinical studies of HIV-1 treatment-naive subjects receiving EVG/COBI/FTC/TAF [abstract no. P7]. HIV Med. 2015;16(Suppl 2):14.
Wohl D, Oka S, Clumeck N, et al. A randomized, double-blind comparison of tenofovir alafenamide (TAF) vs. tenofovir disoproxil fumarate (TDF), each coformulated with elvitegravir, cobicistat, and emtricitabine (E/C/F) for initial HIV-1 treatment: week 96 results. J Acquir Immune Defic Syndr. 2016;72(1):58–64.
Gaur AH, Kizito H, Chakraborty R, et al. Safety and efficacy of E/C/F/TAF in HIV-1 infected treatment-naive adolescents [abstract no. 817 plus poster]. In: CROI 2016.
Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16(1):43–52.
McComsey G, Funderburg N, Kulkarni M, et al. Equivalent decline in inflammation markers with tenofovir alafenamide and tenofovir disoproxil fumarate [abstract no. 717 plus poster]. In: CROI 2016.
Birkus G, Bam RA, Willkom M, et al. Intracellular activation of tenofovir alafenamide and the effect of viral and host protease inhibitors. Antimicrob Agents Chemother. 2016;60(1):316–22.
Birkus G, Wang R, Liu X, et al. Cathepsin A is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131. Antimicrob Agents Chemother. 2007;51(2):543–50.
Gilead Sciences Inc. Viread® (tenofovir disoproxil fumarate) tablets, for oral use: US prescribing information. 2016. http://www.fda.gov. Accessed 27 Apr 2016.
Pozniak A, Arribas JR, Gathe J, et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48-week results from a single-arm, multicenter, open-label, phase 3 study. J Acquir Immune Defic Syndr. 2016;71(5):530–7.
Batra J, Kizito H, Gaur A, et al. Week 24 data from a phase 3 clinical trial of E/C/F/TAF in HIV-positive adolescents [abstract no. P026]. J Int AIDS Soc. 2015;18(Suppl 2):22.
Post FA, Tebas P, Clarke A, et al. Longer-term safety of tenofovir alafenamide in renal impairment [abstract no. 680 plus poster]. In: CROI 2016.
Wohl D, Thalme A, Finlayson R, et al. Renal safety of tenofovir alafenamide in patients at high risk of kidney disease [abstract no. 681 plus poster]. In: CROI 2016.
Gilead Sciences Inc. US Food and Drug Administration approves Gilead’s single tablet regimen Genvoya® (elvitegravir, cobicistat, emtricitabine and tenofovir alafenamide) for treatment of HIV-1 infection [media release]. 5 Nov 2015. http://www.gilead.com/news.
Gilead Sciences Inc. European Commission grants marketing authorization for Gilead’s single tablet regimen Genvoya® (elvitegravir, cobicistat, emtricitabine and tenofovir alafenamide) for the treatment of HIV-1 infection [media release]. 23 Nov 2015. http://www.gilead.com/news.
Gilead Sciences Inc. Odefsey® (emtricitabine, rilpivirine, and tenofovir alafenamide) tablets, for oral use: US prescribing information. 2016. http://www.fda.gov. Accessed 27 Apr 2016.
Gilead Sciences Inc. Descovy® (emtricitabine and tenofovir alafenamide) tablets, for oral use: US prescribing information. 2016. http://www.fda.gov. Accessed 27 Apr 2016.
European Medicines Agency. CHMP summary of positive opinion for Descovy (emtricitabine/tenofovir alafenamide) [media release]. 25 Feb 2016. http://www.ema.europa.eu.
Wyatt CM. Will a new tenofovir prodrug for the treatment of HIV reduce the risk of nephrotoxicity? Kidney Int. 2016;89(1):5–6.
Vitoria M, Hill AM, Ford NP, et al. Choice of antiretroviral drugs for continued treatment scale-up in a public health approach: what more do we need to know? J Int AIDS Soc. 2016;19(1):20504.
Walensky RP, Horn TH, Paltiel AD. The epi-TAF for tenofovir disoproxil fumarate? Clin Infect Dis. 2016;62(7):915–8.
Acknowledgments
During the peer review process, the manufacturer of elvitegravir/cobicistat/emtricitabine/tenofovir AF was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Sarah Greig and Emma Deeks are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: D. M. Burger, Radboud University Medical Centre, Nijmegen, The Netherlands; A. Hill, Pharmacology Research Laboratories, University of Liverpool, Liverpool, UK; A. Mills, Southern California Men’s Medical Group, Los Angeles, CA, USA.
Rights and permissions
About this article
Cite this article
Greig, S.L., Deeks, E.D. Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection. Drugs 76, 957–968 (2016). https://doi.org/10.1007/s40265-016-0586-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0586-z