Abstract
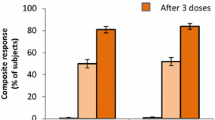
MenB-FHbp (bivalent rLP2086; Trumenba®) is a recombinant protein-based vaccine targeting Neisseria meningitidis serogroup B (MenB), which has recently been licensed in the EU for active immunization to prevent invasive disease caused by MenB in individuals ≥ 10 years of age. The vaccine, which contains a variant from each of the two identified subfamilies of the meningococcal surface protein factor H-binding protein (fHBP), has been licensed in the USA for active immunization in individuals 10–25 years of age since 2014. This article reviews the immunogenicity, reactogenicity and tolerability of MenB-FHbp, with a focus on the EU label and the European setting. As demonstrated in an extensive program of clinical trials in adolescents and young adults, a two-dose or three-dose series of MenB-FHbp elicits a strong immune response against a range of MenB test strains selected to be representative of strains prevalent in Europe and the USA. Follow-up studies investigating the persistence of the MenB-FHbp immune response and the effect of a booster dose of the vaccine indicate that a booster dose should be considered (following a primary vaccine series) in individuals at continued risk of invasive meningococcal disease. MenB-FHbp vaccine appears to be moderately reactogenic but, overall, is generally well tolerated, with most adverse reactions being mild to moderate in severity. Although post-marketing, population-based data will be required to establish the true effectiveness of the vaccine, currently available data indicate that MenB-FHbp, in a two-dose or three-dose series, is likely to provide broad protection against MenB strains circulating in Europe.
Similar content being viewed by others
References
Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal disease. N Engl J Med. 2001;344(18):1378–88.
Jafri RZ, Ali A, Messonnier NE, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11(1):17.
Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30(Suppl 2):B3–9.
European Centre for Disease Prevention and Control. Annual epidemiological report for 2015—invasive meningococcal disease. 2017. http://ecdc.europa.eu/sites/portal/files/documents/AER_for_2015-meningococcal-disease.pdf. Accessed 22 Dec 2017.
Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129(6):1307–26.
Nicholson A, Lepow IH. Host defense against Neisseria meningitidis requires a complement-dependent bactericidal activity. Science. 1979;205(4403):298–9.
Halperin SA, Bettinger JA, Greenwood B, et al. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(Suppl 2):B26–36.
Boisier P, Nicolas P, Djibo S, et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis. 2007;44(5):657–63.
Cohn AC, Harrison LH. Meningococcal vaccines: current issues and future strategies. Drugs. 2013;73(11):1147–55.
Borrow R. Advances with vaccination against Neisseria meningitidis. Trop Med Int Health. 2012;17(12):1478–91.
Feavers IM, Pizza M. Meningococcal protein antigens and vaccines. Vaccine. 2009;27(Suppl 2):B42–50.
Holst J, Martin D, Arnold R, et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009;27(Suppl 2):B3–12.
Fletcher LD, Bernfield L, Barniak V, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun. 2004;72(4):2088–100.
Masignani V, Comanducci M, Giuliani MM, et al. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med. 2003;197(6):789–99.
Madico G, Welsch JA, Lewis LA, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177(1):501–10.
Schneider MC, Prosser BE, Caesar JJE, et al. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature. 2009;458(7240):890–3.
Jiang H-Q, Hoiseth SK, Harris SL, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine. 2010;28(37):6086–93.
McNeil LK, Zagursky RJ, Lin SL, et al. Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease. Microbiol Mol Biol Rev. 2013;77(2):234–52.
McNeil LK, Murphy E, Zhao X-J, et al. Detection of LP2086 on the cell surface of Neisseria meningitidis and its accessibility in the presence of serogroup B capsular polysaccharide. Vaccine. 2009;27(25–26):3417–21.
European Medicines Agency. Trumenba (meningococcal group B vaccine): summary of product characteristics. 2017. http://www.ema.europa.eu. Accessed 22 Dec 2017.
European Medicines Agency. Bexsero (meningococcal group B vaccine): summary of product characteristics. 2017. http://www.ema.europa.eu. Accessed 22 Dec 2017.
Sheldon EA, Schwartz H, Jiang Q, et al. A phase 1, randomized, open-label, active-controlled trial to assess the safety of a meningococcal serogroup B bivalent rLP2086 vaccine in healthy adults. Hum Vaccin Immunother. 2012;8(7):888–95.
Marshall HS, Richmond PC, Nissen MD, et al. A phase 2 open-label safety and immunogenicity study of a meningococcal B bivalent rLP2086 vaccine in healthy adults. Vaccine. 2013;31(12):1569–75.
Richmond PC, Marshall HS, Nissen MD, et al. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12(8):597–607.
Reiner DM, Bhuyan P, Eiden JJ, et al. Immunogenicity, safety, and tolerability of the meningococcal serogroup B bivalent rLP2086 vaccine in adult laboratory workers. Vaccine. 2016;34(6):809–13.
European Medicines Agency. European public assessment report: Trumenba. 2017. http://www.ema.europa.eu. Accessed 22 Dec 2017.
Borrow R, Carlone GM, Rosenstein N, et al. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine. 2006;24(24):5093–107.
Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine. 2009;27(Suppl 2):B112–6.
Donald RGK, Hawkins JC, Hao L, et al. Meningococcal serogroup B vaccines: estimating breadth of coverage. Hum Vaccin Immunother. 2017;13(2):255–65.
Zlotnick GW, Jones TR, Liberator P, et al. The discovery and development of a novel vaccine to protect against Neisseria meningitidis serogroup B disease. Hum Vaccin Immunother. 2015;11(1):5–13.
Ostergaard L, Vesikari T, Absalon J, et al. A bivalent meningococcal B vaccine in adolescents and young adults. N Engl J Med. 2017;377(24):2349–62.
Vesikari T, Østergaard L, Diez-Domingo J, et al. Meningococcal serogroup B bivalent rLP2086 vaccine elicits broad and robust serum bactericidal responses in healthy adolescents. J Pediatric Infect Dis Soc. 2016;5(2):152–60.
Marshall HS, Richmond PC, Beeslaar J, et al. Meningococcal serogroup B-specific responses after vaccination with bivalent rLP2086: 4 year follow-up of a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2017;17(1):58–67.
Vesikari T, Wysocki J, Beeslaar J, et al. Immunogenicity, safety, and tolerability of bivalent rLP2086 meningococcal group B vaccine administered concomitantly with diphtheria, tetanus, and acellular pertussis and inactivated poliomyelitis vaccines to healthy adolescents. J Pediatric Infect Dis Soc. 2016;5(2):180–7.
Senders S, Bhuyan P, Jiang Q, et al. Immunogenicity, tolerability and safety in adolescents of bivalent rLP2086, a meningococcal serogroup B vaccine, coadministered with quadrivalent human papilloma virus vaccine. Pediatr Infect Dis J. 2016;35(5):548–54.
Muse D, Christensen S, Bhuyan P, et al. A phase 2, randomized, active-controlled, observer-blinded study to assess the immunogenicity, tolerability and safety of bivalent rLP2086, a meningococcal serogroup B vaccine, coadministered with tetanus, diphtheria and acellular pertussis vaccine and serogroup A, C, Y and W-135 meningococcal conjugate vaccine in healthy US adolescents. Pediatr Infect Dis J. 2016;35(6):673–82.
Ostergaard L, Lucksinger GH, Absalon J, et al. A phase 3, randomized, active-controlled study to assess the safety and tolerability of meningococcal serogroup B vaccine bivalent rLP2086 in healthy adolescents and young adults. Vaccine. 2016;34(12):1465–71.
Murphy E, Andrew L, Lee K-L, et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis. 2009;200(3):379–89.
Taha M-K, Hawkins JC, Liberator P, et al. Bactericidal activity of sera from adolescents vaccinated with bivalent rLP2086 against meningococcal serogroup B outbreak strains from France. Vaccine. 2017;35(11):1530–7.
Harris SL, Donald RGK, Hawkins JC, et al. Neisseria meningitidis serogroup B vaccine, bivalent rLP2086, induces broad serum bactericidal activity against diverse invasive disease strains including outbreak strains. Pediatr Infect Dis J. 2017;36(2):216–23.
Rosain J, Hong E, Fieschi C, et al. Strains responsible for invasive meningococcal disease in patients with terminal complement pathway deficiencies. J Infect Dis. 2017;215(8):1331–8.
Ala’Aldeen DAA, Flint M, Oldfield NJ, et al. Human antibody responses to the meningococcal factor H binding protein (LP2086) during invasive disease, colonization and carriage. Vaccine. 2010;28(48):7667–75.
Lemée L, Hong E, Etienne M, et al. Genetic diversity and levels of expression of factor H binding protein among carriage isolates of Neisseria meningitidis. PLoS One. 2014;9(9):e107240.
Soeters HM, Whaley M, Alexander-Scott N, et al. Meningococcal carriage evaluation in response to a serogroup B meningococcal disease outbreak and mass vaccination campaign at a college—Rhode Island, 2015–2016. Clin Infect Dis. 2017;64(8):1115–22.
Read RC, Baxter D, Chadwick DR, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384(9960):2123–31.
Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-2):1–28.
Maiden MCJ, Ibarz-Pavón AB, Urwin R, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197(5):737–43.
Anderson AS, Jansen KU, Eiden J. New frontiers in meningococcal vaccines. Expert Rev Vaccines. 2011;10(5):617–34.
Anderson AS, Hao L, Jiang Q, et al. Potential impact of the bivalent rLP2806 vaccine on Neisseria meningitidis carriage and invasive serogroup B disease. Hum Vaccin Immunother. 2013;9(3):471–9.
Wang X, Cohn A, Comanducci M, et al. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the United States. Vaccine. 2011;29(29–30):4739–44.
Beernink PT, Caugant DA, Welsch JA, et al. Meningococcal factor H-binding protein variants expressed by epidemic capsular group A, W-135, and X strains from Africa. J Infect Dis. 2009;199(9):1360–8.
Harris SL, Zhu D, Murphy E, et al. Preclinical evidence for the potential of a bivalent fHBP vaccine to prevent Neisseria menigitidis serogroup C disease. Hum Vaccin. 2011;7(Suppl 1):68–74.
Ladhani SN, Giuliani MM, Biolchi A, et al. Effectiveness of meningococcal B vaccine against endemic hypervirulent Neisseria meningitidis W strain, England. Emerg Infect Dis. 2016;22(2):309–11.
Hong E, Giuliani MM, Deghmane A-E, et al. Could the multicomponent meningococcal serogroup B vaccine (4CMenB) control Neisseria meningitidis capsular group X outbreaks in Africa? Vaccine. 2013;31(7):1113–6.
Harris SL, Tan C, Andrew L, et al. Bivalent rLP2086 elicits bactericidal antibodies against non-serogroup B [abstract no. ESP17-1236]. In: 35th Annual Meeting of the European Society for Paediatric Infectious Diseases. 2017.
European Centre for Disease Prevention and Control. Vaccine schedule. 2017. http://vaccine-schedule.ecdc.europa.eu. Accessed 22 Dec 2017.
MacNeil JR, Rubin L, Folaranmi T, et al. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(41):1171–6.
Crum-Cianflone N, Sullivan E. Meningococcal vaccinations. Infect Dis Ther. 2016;5(2):89–112.
Gasparini R, Amicizia D, Lai PL, et al. Meningococcal B vaccination strategies and their practical application in Italy. J Prev Med Hyg. 2015;56(3):E133–9.
Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥ 10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(22):608–12.
Patton ME, Stephens D, Moore K, et al. Updated recommendations for use of MenB-FHbp serogroup B meningococcal vaccine—Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(19):509–13.
Donnelly J, Medini D, Boccadifuoco G, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci USA. 2010;107(45):19490–5.
Plikaytis BD, Stella M, Boccadifuoco G, et al. Interlaboratory standardization of the sandwich enzyme-linked immunosorbent assay designed for MATS, a rapid, reproducible method for estimating the strain coverage of investigational vaccines. Clin Vaccin Immunol. 2012;19(10):1609–17.
Vogel U, Taha M-K, Vazquez JA, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013;13(5):416–25.
Frosi G, Biolchi A, Lo Sapio M, et al. Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage. Vaccine. 2013;31(43):4968–74.
Acknowledgements
During the peer review process, the manufacturer of MenB-FHbp (Trumenba®) was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Matt Shirley is a salaried employee of Adis/Springer and declares no relevant conflicts of interest. Muhamed-Kheir Taha performs contract work for the Institut Pasteur funded by GSK, Pfizer and Sanofi Pasteur.
Additional information about this Adis Drug Review can be found at: http://www.medengine.com/Redeem/6B4CF0604CD4F8A4.
Additional information
The manuscript was reviewed by: R. Borrow, Vaccine Evaluation Unit, Public Health England, Manchester, UK; M. Petráš, Third Faculty of Medicine, Charles University, Prague, Czech Republic.
Rights and permissions
About this article
Cite this article
Shirley, M., Taha, MK. MenB-FHbp Meningococcal Group B Vaccine (Trumenba®): A Review in Active Immunization in Individuals Aged ≥ 10 Years. Drugs 78, 257–268 (2018). https://doi.org/10.1007/s40265-018-0869-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0869-7