Abstract
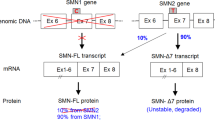
Onasemnogene abeparvovec (onasemnogene abeparvovec-xioi; formerly AVXS-101; ZOLGENSMA®) is an adeno-associated viral vector-based gene therapy designed to deliver a functional copy of the human survival motor neuron (SMN) gene to the motor neuron cells of patients with spinal muscular atrophy (SMA). It has been developed by AveXis, a Novartis company, and was approved in May 2019 in the USA for the treatment of paediatric patients aged < 2 years with SMA and bi-allelic mutations in the SMN1 gene (the primary gene encoding survival motor neuron protein). Onasemnogene abeparvovec is the first gene therapy to be approved for SMA in the USA. The recommended dose is 1.1 × 1014 vector genomes per kg of bodyweight, administered as a single intravenous infusion over 60 min. Regulatory assessments for this formulation of onasemnogene abeparvovec are underway in the EU and Japan; an intrathecal formulation is currently undergoing clinical development in the USA. This article summarizes the milestones in the development of onasemnogene abeparvovec leading to this first approval for the treatment of paediatric patients aged < 2 years with SMA and bi-allelic mutations in SMN1.
Similar content being viewed by others
References
US National Library of Medicine. Genetics Home Reference: spinal muscular atrophy. 2019. https://ghr.nlm.nih.gov/condition/spinal-muscular-atrophy. Accessed 6 Jun 2019.
Groen EJN, Talbot K, Gillingwater TH. Advances in therapy for spinal muscular atrophy: promises and challenges. Nat Rev Neurol. 2018;14(4):214–24.
Verhaart IEC, Robertson A, Wilson IJ, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy: a literature review. Orphanet J Rare Dis. 2017;12(1):124.
Shorrock HK, Gillingwater TH, Groen EJN. Overview of current drugs and molecules in development for spinal muscular atrophy therapy. Drugs. 2018;78(3):293–305.
Feldkötter M, Schwarzer V, Wirth R, et al. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70(2):358–68.
AveXis Inc. New AveXis data at AAN showed long-term durability of Zolgensma® in patients with spinal muscular atrophy (SMA) type 1 [media release]. http://www.avexis.com/. Accessed 7 May 2019.
National Institute for Health Research. AVXS-101 for spinal muscular atrophy. 2018. http://www.io.nihr.ac.uk/report/avxs-101-for-spinal-muscular-atrophy/. Accessed 7 Jun 2019.
AveXis Inc. ZOLGENSMA® (onasemnogene abeparvovec-xioi): US prescribing information. 2019. http://www.fda.gov/vaccines-blood-biologics/zolgensma. Accessed 27 May 2019.
US Food and Drug Administration. FDA approves innovative gene therapy to treat pediatric patients with spinal muscular atrophy, a rare disease and leading genetic cause of infant mortality [media release]. http://www.fda.gov/. Accessed 24 May 2019.
AveXis Inc. AveXis presented robust data at AAN demonstrating efficacy of Zolgensma® in broad spectrum of spinal muscular atrophy (SMA) patients [media release]. http://www.avexis.com/. Accessed 5 May 2019.
Asklepios BioPharmaceutical. Asklepios and AveXis finalize license [media release]. http://www.askbio.com. Accessed 17 Jun 2015.
AveXis Inc. AveXis enters into licensing agreement with Genethon [media release]. http://www.avexis.com. Accessed 13 May 2018.
Novartis. Novartis successfully completes acquisition of AveXis, Inc. [media release]. http://www.novartis.com. Accessed 15 May 2018.
Regenxbio. REGENXBIO receives $100 Million accelerated license payment due to acquisition of AveXis by Novartis [media release]. http://www.regenxbio.com. Accessed 11 Jun 2018.
Regenxbio, AveXis Inc. REGENXBIO and AveXis announce expansion of relationship through amended license agreement for the development and commercialization of treatments for spinal muscular atrophy [media release]. http://www.regenxbio.com. Accesssed 8 Jan 2018.
Foust KD, Nurre E, Montgomery CL, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27(1):59–65.
Bevan AK, Duque S, Foust KD, et al. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther J Am Soc Gene Ther. 2011;19(11):1971–80.
Foust KD, Wang X, McGovern VL, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28(3):271–4.
Foust K, Meyer K, Likhite S, et al. Extensive dose ranging study of AVXS-101 in the severe D7 SMA mouse model [abstract no. 435]. Mol Ther. 2017;25(5 Suppl 1):200–1.
AveXis Inc. AveXis data reinforce effectiveness of Zolgensma® in treating spinal muscular atrophy (SMA) type 1 [media release]. http://www.avexis.com. Accessed 15 Apr 2019.
Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713–22.
Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810–7.
Lowes LP, Alfano LN, Arnold WD, et al. Impact of age and motor function in a phase 1/2A study of infants with SMA type 1 receiving single-dose gene replacement therapy. Pediatr Neurol. 2019. https://doi.org/10.1016/j.pediatrneurol.2019.05.005.
Dabbous O, Maru B, Jansen JP, et al. Survival, motor function, and motor milestones: comparison of AVXS-101 relative to nusinersen for the treatment of infants with spinal muscular atrophy type 1. Adv Ther. 2019;36(5):1164–76.
Malone DC, Dean R, Arjunji R, et al. Cost-effectiveness analysis of using onasemnogene abeparvocec (AVXS-101) in spinal muscular atrophy type 1 patients. J Mark Access Health Policy. 2019;7(1):1601484.
Mendell JR, Al-Zaidy S, Shell R, et al. AVXS-101 phase 1 gene replacement therapy clinical trial in spinal muscular atrophy type 1 (SMA1): 24-month event-free survival and achievement of developmental milestones [abstract no. OR005]. Hum Gene Ther. 2018;29(12):A22.
Al-Zaidy S, Pickard AS, Kotha K, et al. Health outcomes in spinal muscular atrophy type 1 following AVXS-101 gene replacement therapy. Pediatr Pulmonol. 2019;54(2):179–85.
US FDA. ZOLGENSMA® (onasemnogene abeparvovec-xioi): summary basis for regulatory action. 2019. http://www.fda.gov/vaccines-blood-biologics/zolgensma. Accessed 26 Jun 2019.
Mendell JR, Lehman KJ, McColly M, et al. AVXS-101 gene-replacement therapy (GRT) in spinal muscular atrophy type 1 (SMA1): long-term follow-up from the phase 1 clinical trial [abstract no. S25.006 plus presentation]. Neurology. 2019;92(15 Suppl).
Day JW, Chiriboga CA, Crawford TO, et al. AVXS-101 gene-replacement therapy (GRT) for spinal muscular atrophy type 1 (SMA1): pivotal phase 3 study (STR1VE) update [abstract no. P1.6-058 plus poster]. Neurology. 2019;92(15 Suppl).
Finkel RS, Day JW, Darras BT, et al. Phase 1 study of intrathecal administration of AVXS-101 gene-replacement therapy (GRT) for spinal muscular atrophy type 2 (SMA2) (STRONG) [abstract no. P1.6-059 plus poster]. Neurology. 2019;92(15 Suppl).
Schultz M, Swoboda K, Farrar M, et al. AVXS-101 gene-replacement therapy (GRT) in presymptomatic spinal muscular atrophy (SMA): study update [abstract no. P1.6-057 plus poster]. Neurology. 2019;92(15 Suppl).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Sheridan Hoy is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Additional information for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.8323529.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Hoy, S.M. Onasemnogene Abeparvovec: First Global Approval. Drugs 79, 1255–1262 (2019). https://doi.org/10.1007/s40265-019-01162-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01162-5