Abstract
Background
The impact of statins on COVID-19 outcomes is important given the high prevalence of their use among individuals at risk for severe COVID-19. Our aim is to assess whether patients receiving chronic statin treatment who are hospitalized with COVID-19 have reduced in-hospital mortality if statin therapy is maintained during hospitalization.
Methods
This work is a cross-sectional, observational, retrospective multicenter study that analyzed 2921 patients who required hospital admission at 150 Spanish centers included in the nationwide SEMI-COVID-19 Network. We compared the clinical characteristics and COVID-19 disease outcomes between patients receiving chronic statin therapy who maintained this therapy during hospitalization versus those who did not. Propensity score matching was used to match each statin user whose therapy was maintained during hospitalization to a statin user whose therapy was withdrawn during hospitalization.
Results
After propensity score matching, continuation of statin therapy was associated with lower all-cause mortality (OR 0.67, 0.54–0.83, p < 0.001); lower incidence of acute kidney injury (AKI) (OR 0.76,0.6–0.97, p = 0.025), acute respiratory distress syndrome (ARDS) (OR 0.78, 0.69- 0.89, p < 0.001), and sepsis (4.82% vs 9.85%, p = 0.008); and less need for invasive mechanical ventilation (IMV) (5.35% vs 8.57, p < 0.001) compared to patients whose statin therapy was withdrawn during hospitalization.
Conclusions
Patients previously treated with statins who are hospitalized for COVID-19 and maintain statin therapy during hospitalization have a lower mortality rate than those in whom therapy is withdrawn. In addition, statin therapy was associated with a decreased probability that patients with COVID-19 will develop AKI, ARDS, or sepsis and decreases the need for IMV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Prior statin therapy that is maintained during hospitalization is associated with lower mortality rate in patients hospitalized for COVID-19. |
Prior statin therapy that is maintained during hospitalization is associated with lower probability of AKI and sepsis rate in patients hospitalized for COVID-19. |
Prior statin therapy that is maintained during hospitalization is associated with lower probability of ARDS and IMV rate in patients hospitalized for COVID-19. |
1 Introduction
SARS-Cov-2 (severe acute respiratory syndrome coronavirus 2) infection is a rapidly evolving pandemic with uncertain clinical features. Its true burden on the healthcare system may be underestimated since extrapulmonary manifestations are frequent. Patients with pre-existing cardiovascular disease may develop clinical events early on in the course of the disease and the infection may also have short-, medium-, and long-term implications for cardiovascular health—a finding which has been observed in several patient registries [1,2,3,4].
Whether vascular disorders in patients with COVID-19 (coronavirus disease-2019), the disease caused by SARS-CoV-2, are due to direct involvement of the virus on endothelial cells is not currently known. Endothelial dysfunction is a main determinant in the development of arteriosclerotic cardiovascular disease [5, 6]. Microvascular endothelial dysfunction occurs when there is an imbalance between vasoconstriction and vasodilation in favor of vasoconstriction, resulting in organ ischemia, inflammation with edema of the associated tissues, and a procoagulant state. Recent data support the hypothesis of a direct cytotoxic effect of SARS-CoV-2 on endothelial cells and that this contributes to diffuse endothelial inflammation [7]. These findings show the presence of viral elements within endothelial cells and an accumulation of inflammatory cells, with evidence of inflammation and endothelial cell death. Furthermore, the induction of apoptosis and pyroptosis phenomena may play an important role in endothelial cell injury in patients with COVID-19 [7]. In this sense, severe endothelial damage associated with the presence of intracellular viral particles with rupture of endothelial cell membranes has recently been described in a small series of autopsies in deceased COVID-19 patients, together with histological findings showing thrombosis and microangiopathy [8].
Statins improve endothelial dysfunction by decreasing levels of plasma cholesterol and increasing endothelial nitric oxide (NO) synthesis, stimulating and regulating the action of endothelial NO synthase [9]. They also have anti-inflammatory and immunomodulatory properties, antithrombotic and antiproliferative actions, and reduce the rate of apoptosis [10].
It is known that lipid rafts rich in cholesterol serve as docking SARS-CoV-2 infection. Lipid rafts rich in cholesterol serve as docking sites in SARS-COV-2 infection and for angiotensin-converting enzyme 2 (ACE2) receptors and viral attachment via the S protein sites for ACE2 receptors and viral attachment via the S protein of SARS-CoV-2, and then is taken into the cells by clathrin. Moreover, SARS-COV-2 infection and macrophages can lead to plaque instability and embolization by paracrine pathway. Thus, statins can disrupt lipid rafts and viral binding, modulating viral entry by reducing cholesterol and improving plaque stability, antithrombotic, and anti-inflammatory properties [11]. To sum up, the pharmacological sequestration of cellular or viral cholesterol with statins may significantly blocked both virus attachment and internalization [12]. Antiviral effects of statins has been also proposed, suggesting that statins may have a role in the treatment of viral infections due to their immunomodulatory properties and the inhibition of viral replication acting in different stages of virus cell cycle [13].
For all of these reasons, it would be interesting to know the role of statin therapy in the stabilization process of endothelial cells while viral replication is taking place, especially given their known anti-inflammatory, immunomodulatory, antithrombotic, and antiproliferative properties. Thus, the aim of this work is to assess whether hospitalized patients with COVID-19 in chronic treatment with statins have lower in-hospital mortality and other COVID-19 outcomes if their statin therapy is maintained during the hospitalization.
2 Materials and Methods
2.1 Source of Data
This is a multicenter, retrospective, cohort study based on the SEMI-COVID-19 Registry. This registry includes consecutive patients with COVID-19 infection confirmed via a positive reverse transcription polymerase chain reaction (RT-PCR) test who are hospitalized in 150 Spanish hospitals. Clinical and epidemiological data, laboratory tests upon admission and at 7 days of hospitalization, treatments administered, complications, and their status upon discharge and at 30 days after diagnosis were recorded in electronic medical records and compiled in a secure database. Patients aged < 18 years and patients who did not agree to participate were excluded. The study was approved by the Research Ethics Committee of Málaga (Spain). Further information on the justification, objectives, and methodology of the SEMI-COVID-19 registry has recently been published [4].
2.2 Outcomes
The primary outcome was all-cause in-hospital mortality expressed as the case fatality rate: the proportion of deaths during hospitalization compared to the total number of hospitalized patients with COVID-19. Secondary outcomes were the length of hospital stay and in-hospital complications, including acute respiratory distress syndrome (ARDS), need for invasive mechanical ventilation (IMV), sepsis, and acute kidney injury (AKI).
2.3 Data Analysis
Participants’ demographic, clinical, epidemiological, laboratory, and diagnostic imaging data were analyzed. Treatment received, complications, and clinical progress were also examined. Quantitative variables were expressed as means and SD or median and interquartile range. Continuous variables were tested for normal distribution using Kolmogorov–Smirnov. Categorical variables were expressed as absolute frequencies and percentages. P values were obtained using the chi-square test, Fisher’s exact test, or Mann-Whitney U test, when appropriate. Two-tailed p value < 0.05 was considered statistically significant.
2.4 Statistical Analysis
Propensity score matching was performed to account for non-randomized treatment decisions and reduce the effects of confounding variables. Logistic regression was used to determine the probability of having statin treatment and included confounding variables that could have affected treatment choice (age, sex, obesity, hypertension, diabetes, coronary artery disease, dyslipidemia, ischemic stroke, transient ischemic attack, peripheral artery disease, heart failure, treatment with angiotensin-converting enzyme inhibitors, chronic kidney disease; angiotensin II receptor blockers, qSOFA category, C-reactive protein, D-dimer, lymphocyte count, and serum creatinine). The nearest neighbor method with a caliper of 0.1 was used in the propensity score matching and standardized mean differences (SMD) were calculated to evaluate the adequacy of propensity matching.
In order to estimate the association of statin treatment (specifically, in-hospital use of statins during hospitalization vs stopping statin treatment in patients with prior statin treatment) on mortality and other endpoints, both conditional and mixed-effects logistic regressions were performed considering matched pairs as random effects. For the sepsis and IMV endpoints, McNemar test was used to observe differences between the treatments. Statistical analyses were performed using R software, version 3.6.2 (The R Foundation for Statistical Computing, http://www.R-project.org).
2.5 Ethical Aspects
The SEMI-COVID-19 Registry has been approved by the Provincial Research Ethics Committee of Málaga (Spain). Informed consent was obtained from all patients. When it was not possible to obtain informed consent in writing due to biosafety concerns, informed consent was requested verbally and noted on the medical record.
3 Results
3.1 Baseline Clinical Variables
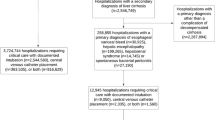
Figure 1 shows the flowchart for patient inclusion. Table 1 shows the baseline demographic characteristics, and complications of all patients included in this sub-analysis. A total of 2921 patients had prior statin therapy at the time of inclusion in the registry and 6669 patients did not. Overall, there were no significant differences between the two groups in the main variables analyzed except for the higher rate of coronary artery disease among statin users.
3.2 Pre- and Post-propensity Score Matching Characteristics in Patient Treated with Statins before Admission
Pre- and post-propensity score matching was used to compare two subgroups of patients within the prior statin use group: those who continued to receive statin therapy during their hospitalization and those who did not. The results of the comparison of their baseline sociodemographic and clinical characteristics are shown in Table 2. After propensity score matching, the subgroups were well-balanced.
3.3 Association between Statin Therapy on Study Outcomes
Tables 3 and 4 show the main clinical outcomes of the study after propensity score matching. Upon analyzing the two subgroups of patients within the prior statin use group (those who continued to receive statin therapy during hospitalization and those who did not), continuation of statin therapy was associated with lower all-cause mortality (OR 0.67, 0.54–0.83, p < 0.001), a lower incidence of AKI (OR 0.76, 0.6–0.97, p = 0.025), and a lower incidence of ARDS (OR 0.78, 0.69–0.89, p < 0.001) (Table 3). In addition, fewer patients in this subgroup required IMV (5.35 % vs 8.57 %, p < 0.001) and there was a lower incidence of sepsis (4.82 % vs 9.85 %, p = 0.008) compared to patients who did not continue to receive statin therapy during the hospitalization (Table 4).
4 Discussion
This work shows that patients previously treated with statins who develop COVID-19 and continue to receive statins during their hospitalization were associated with a lower mortality rates than those in whom statins were withdrawn. Also, continued in-hospital statin use was associated with a decreased probability of developing other in-hospital complications, including ARDS, a need for IMV, sepsis, or AKI. Our results are consistent with other studies that suggest a potential beneficial role of statins in patients with COVID-19.
Recently, a retrospective cohort study by Zhang et al. [14] reported a significantly lower in-hospital death rate among patients with in-hospital statin use compared to a matched non-statin-use control group (5.2 % vs 9.4 %). A meta-analysis that included four studies of nearly 9000 patients with severe COVID-19 [15], including three large-scale studies that adjusted for multiple confounding variables, found that patients taking statins had a 30 % lower risk of death or serious disease when compared to those not taking statins (pooled HR 0.70; 95 % CI 0.53–0.94). Also, work by Daniels et al. [16] showed that among patients hospitalized for COVID-19, statin use prior to admission was associated with a reduced risk of severe disease (death or intensive care unit admission) and a faster recovery time. Specific populations may benefit from the use of statins in the context of COVID-19 [17], thus, an observational study showed that statin therapy was associated with reduced in-hospital mortality from COVID-19 in patients with diabetes. All these findings suggest that statins may favorably modulate COVID-19 disease outcomes.
It has previously been theorized that the use of statins could reduce the probability of complications and mortality in patients with COVID-19; in fact, statins have even been included as a possible anti-COVID-19 therapy in some guidelines [18]. Evidence suggests that statins exert antiviral activity and could block the infectivity of encapsulated viruses [19]. The main protease of SARS-CoV-2 called Mpro—a key enzyme in the coronavirus—has recently come into focus and is a possible pharmacological target. Using a molecular coupling model, it has been shown that statins can be efficient inhibitors of this enzyme [20] and therefore could be used as a potential treatment against SARS-CoV-2.
On the other hand, toll-like receptors (TLR) have been shown to intervene in the immune response mediated by activation of the NF-KB signaling pathway [21]. Activation of TLR and NF-KB by coronaviruses has been observed to trigger both over-expression and under-expression of the MyD88 gene (involved in the expression of myeloid differentiation factors) in experimental mouse models, which has been associated with an increased mortality after MERS-CoV infection [21]. Due to their potential effect of stopping TLR and NF-KB signaling, statins may improve the lung damage associated with SARS-CoV-2 infection through these anti-inflammatory effects. It is important to note that pharmacokinetic characteristics may be relevant in patients who receive statins in the context of COVID-19. Rossi et al. [22] hypothesized that patients taking statins were better protected against mortality than those who do not take statins. Interestingly, they observed that in the group receiving lipophilic statins, mortality was significantly lower compared to patients who did not take statins and those who received hydrophilic statins.
Statins have also been reported to reduce the risk of COVID-19-induced acute coronary syndrome by stabilizing arteriosclerotic plaques and in turn preventing AKI. Given that acute myocardial injury and AKI are predictors of COVID-19-induced mortality [23], statin therapy may prevent these complications and thus increase survival among patients who receive it. This association has also been found in our registry: patients with COVID-19 who were previously receiving chronic statin treatment had a lower risk of developing AKI. Another potential mechanism by which statins may exert these clinical benefits in patients with COVID-19 is through a significant reduction in cholesterol levels. This reduction may suppress coronavirus infection in various ways. In studies on porcine deltacoronavirus and on the coronavirus that produces infectious bronchitis [12], it has been shown that the reduction in cholesterol, as a result of statin therapy, disrupts the lipid core of the viral envelope, an important element that allows for the binding of the coronavirus to host cells and, consequently, additional infection. Therefore, the action of statins pharmacologically “sequestering” cellular or viral cholesterol served to significantly block virus connection and internalization [12],
All these mechanisms [24] suggest that statins, as anti-inflammatories, play a critical role in inhibiting coronavirus infection due to their effects on the vascular endothelium. For all of these reasons, they can be considered useful drugs to include in the arsenal of anti-COVID-19 therapies.
It is important to highlight that a quarter of our population are patients with type 2 diabetes. The underlying mechanisms involved in endothelial dysfunction when diabetes is present are complex and related to hyperglycemia and insulin resistance [25]. In this context, it is known that statins may exert a protective action on vascular endothelial cells in patients with diabetes [26] through modulation of NO availability, suppression of inflammatory response, prevention of endothelial barrier dysfunction, improvement of plaque stability, and reduced thrombogenic potential of the endothelial cell [27, 28]. This way, statins may favorably modulate the endothelial function in patients with diabetes and COVID-19 disease.
Our findings are important because they provide valuable information on the role of statins during hospitalizations on adverse outcomes in patients admitted for COVID-19. Furthermore, data were collected in a large multicenter, nationwide study. Nevertheless, these results should be considered within the context of some limitations. Our study has several limitations. First, it should be noted that although the sample size is large, this is a retrospective study. Thus, it serves to generate hypotheses that must be verified in future research and randomized clinical trials. Second, important information regarding the statin therapy that patients received (drug, doses, duration of therapy or discontinuation of oral treatment in critically ill patients) or lipid profile was not recorded in the registry. It is obvious that the duration of treatment, potency, and type of statin that patients were receiving may influence outcomes and must be considered.
One relevant limitation is that drug-drug interactions may explain why patients stopped statins during hospitalization. Familial Hypercholesterolemia Europe and the International Lipid Expert Panel (ILEP) in a recent brief report recommended that lipid-lowering drugs are generally safe in patients with coronavirus infections and should be continued. When COVID-19 is treated with antiretroviral drugs it is recommended that prescribers discontinue atorvastatin and simvastatin. It is possible to continue therapy with rosuvastatin, with preference for starting at a low dose and titrating up. Moreover, it is possible to continue treatment with pravastatin or fluvastatin. Caution is necessary when treating patients with macrolides. However, there are no data on severe or serious interactions of rosuvastatin and fluvastatin with azithromycin [29]. Finally, we have data on the administration of contrast that may have influenced the development of contrast-induced acute renal injury.
5 Conclusion
This work shows that patients previously treated with statins (chronic treatment) who develop COVID-19 and continue to receive statins during their hospitalization are associated with a lower mortality rate than those in whom statins were withdrawn. Also, continued in-hospital statin use was associated with a decreased probability of developing other in-hospital complications, including ARDS, a need for IMV, sepsis, or AKI. These findings support a potential role of statin therapy in treating COVID-19. Further prospective studies and randomized controlled clinical trials are needed to determine the effects of statin treatment on COVID-19 outcomes and establish the mechanisms through which statins exert these beneficial effects.
References
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–62. https://doi.org/10.1016/S0140-6736(20)30566-3.
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017.
Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:35. https://doi.org/10.22037/aaem.v8i1.600.
Rojo J, Santos J, Núñez-Cortés J, Bermejo C, Rincón J, Roy Vallejo E, et al. Clinical characteristics of patients hospitalized with COVID-19 in Spain: results from the SEMI-COVID-19 Network. 2020. https://doi.org/10.1101/2020.05.24.20111971.
Reriani MK, Dunlay SM, Gupta B, West CP, Rihal CS, Lerman LO, et al. Effects of statins on coronary and peripheral endothelial function in humans: a systematic review and meta-analysis of randomized controlled trials. Eur J Cardiovasc Prev Rehabil. 2011;18:704–16. https://doi.org/10.1177/1741826711398430.
Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–7. https://doi.org/10.1056/NEJM199502233320801.
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–8. https://doi.org/10.1016/S0140-6736(20)30937-5.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2015432.
Tuñón J, Egido J. endothelial dysfunction, inflamation and statins: new evidences. Revspacardiol. 2004. https://doi.org/10.1157/13066448.
Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5:378–87. https://doi.org/10.1111/j.1582-4934.2001.tb00172.x.
Radenkovic D, Chawla S, Pirro M, Sahebkar A, Banach M. Cholesterol in relation to COVID-19: should we care about It? J Clin Med. 2020. https://doi.org/10.3390/jcm9061909.
Katsiki N, Banach M, Mikhailidis D. Lipid-lowering therapy and renin-angiotensin-aldosterone system inhibitors in the era of the COVID-19 pandemic. Arch Med Sci. 2020;16:485–9. https://doi.org/10.5114/aoms.2020.94503.
Gorabi AM, Kiaie N, Bianconi V, Jamialahmadi T, Al-Rasadi K, Johnston TP, et al. Antiviral effects of statins. Prog Lipid Res. 2020;79:101054. https://doi.org/10.1016/j.plipres.2020.101054.
Zhang X-J, Qin J-J, Cheng X, Shen L, Zhao Y-C, Yuan Y, et al. In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19. Cell Metab. 2020;S1550–4131(20):30316–8. https://doi.org/10.1016/j.cmet.2020.06.015.
Kow CS, Hasan SS. Meta-analysis of Effect of Statins in Patients with COVID-19. Am J Cardiol. https://doi.org/10.1016/j.amjcard.2020.08.004.
Daniels LB, Sitapati AM, Zhang J, Zou J, Bui QM, Ren J, et al. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am J Cardiol. 2020. https://doi.org/10.1016/j.amjcard.2020.09.012.
Omar S, Francesco C, Ilir A, Xiaonan X, Snehal SR, Yogita R, et al. Statin use and in‐hospital mortality in diabetics with COVID‐19. J Am Heart Assoc 15: 475. Doi: https://doi.org/10.1161/JAHA.120.018475.
Massachusetts General Hospital COVID-19 Treatment Guidance Version 1.0 3/17/2020. https://medtube.net/infectious-diseases/medical-documents/26086-covid19-treatmentguidelines-by-massachusetts-general-hospital. Accessed 28 Mar 2020. n.d.
Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto S. Potential inhibitor of COVID-19 Main protease (Mpro) from several medicinal plant compounds by Molecular Docking Study 2020. https://doi.org/10.20944/preprints202003.0226.v1.
Reiner Z, Hatamipour M, Banach M, Pirro M, Al Rasadi K, Jamialahmadi T, et al. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch Med Sci. 2020. https://doi.org/10.5114/aoms.2020.94655.
Dashti-Khavidaki S, Khalili H. Considerations for Statin Therapy in Patients with COVID-19. Pharmacother JHum Pharmacol Drug Ther. 2020;40:484–6. https://doi.org/10.1002/phar.2397.
Rossi R, Talarico M, Coppi F, Boriani G. Protective role of statins in COVID 19 patients: importance of pharmacokinetic characteristics rather than intensity of action. Intern Emerg Med. 2020. https://doi.org/10.1007/s11739-020-02504-y.
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020. https://doi.org/10.1007/s00392-020-01626-9.
Lee KCH, Sewa DW, Phua GC. Potential role of statins in COVID-19. Int J Infect Dis. 2020;96:615–7. https://doi.org/10.1016/j.ijid.2020.05.115.
Lüscher TF, Creager MA, Beckman JA, Francesco C. Diabetes and vascular disease. Circulation. 2003;108:1655–61. https://doi.org/10.1161/01.CIR.0000089189.70578.E2.
Tomizawa A, Hattori Y, Suzuki K, Okayasu T, Kase H, Satoh H, et al. Effects of statins on vascular endothelial function in hypercholesterolemic patients with type 2 diabetes mellitus: fluvastatin vs rosuvastatin. Int J Cardiol. 2010;144:108–9. https://doi.org/10.1016/j.ijcard.2008.12.146.
Haas MJ, Horani MH, Parseghian SA, Mooradian AD. Statins prevent dextrose-induced endothelial barrier dysfunction, possibly through inhibition of superoxide formation. Diabetes. 2006;55:474. https://doi.org/10.2337/diabetes.55.02.06.db05-1078.
Hamilton SJ, Watts GF. Endothelial dysfunction in diabetes: pathogenesis, significance, and treatment. Rev Diabet Stud. 2013;10:133–56. https://doi.org/10.1900/RDS.2013.10.133.
Banach M, Penson PE, Fras Z, Vrablik M, Pella D, Reiner Ž, et al. Brief recommendations on the management of adult patients with familial hypercholesterolemia during the COVID-19 pandemic. Pharmacol Res. 2020;158:104891. https://doi.org/10.1016/j.phrs.2020.104891.
Acknowledgments
We gratefully acknowledge all the investigators who participate in the SEMI-COVID-19 Registry. We also thank the SEMI-COVID-19 Registry Coordinating Center, S&H Medical Science Service, for their quality control data, logistic and administrative support. List of the SEMI-COVID-19 Network members: SEMI-COVID-19 Scientific Committee Members: José Manuel Casas Rojo, José Manuel Ramos Rincón, Carlos Lumbreras Bermejo, Jesús Millán Núñez-Cortés, Juan Miguel Antón Santos, Ricardo Gómez Huelgas. SEMI-COVID-19 Registry Coordinating Center: S & H Medical Science Service. José Manuel Casas Rojo, José Manuel Ramos Rincón, Carlos Lumbreras Bermejo, Jesús Millán Núñez-Cortés, Juan Miguel Antón Santos, Ricardo Gómez Huelgas, Paloma Agudo de Blas, Coral Arévalo Cañas, Blanca Ayuso, José Bascuñana Morejón, Samara Campos Escudero, María Carnevali Frías, Santiago Cossio Tejido, Borja de Miguel Campo, Carmen Díaz Pedroche, Raquel Diaz Simon, Ana García Reyne, Lucia Jorge Huerta, Antonio Lalueza Blanco, Jaime Laureiro Gonzalo, Jaime Lora-Tamayo, Carlos Lumbreras Bermejo, Guillermo Maestro de la Calle, Barbara Otero Perpiña, Diana Paredes Ruiz, Marcos Sánchez Fernández, Javier Tejada Montes, Xavier Corbella, Narcís Homs, Abelardo Montero, Jose María Mora-Luján, Manuel Rubio Rivas, Laura Abarca Casas, Álvaro Alejandre de Oña, Rubén Alonso Beato, Leyre Alonso Gonzalo, Jaime Alonso Muñoz, Crhistian Mario Amodeo Oblitas, Cristina Ausín García, Marta Bacete Cebrián, Jesús Baltasar Corral, Maria Barrientos Guerrero, Alejandro Bendala Estrada, María Calderón Moreno, Paula Carrascosa Fernández, Raquel Carrillo, Sabela Castañeda Pérez, Eva Cervilla Muñoz, Agustín Diego Chacón Moreno, Maria Carmen Cuenca Carvajal, Sergio de Santos, Andrés Enríquez Gómez, Eduardo Fernández Carracedo, María Mercedes Ferreiro-Mazón Jenaro, Francisco Galeano Valle, Alejandra Garcia, Irene Garcia Fernandez-Bravo, María Eugenia García Leoni, Maria Gomez Antunez, Candela González San Narciso, Anthony Alexander Gurjian, Lorena Jiménez Ibáñez, Cristina Lavilla Olleros, Cristina Llamazares Mendo, Sara Luis García, Víctor Mato Jimeno, Clara Millán Nohales, Jesús Millán Núñez-Cortés, Sergio Moragón Ledesma, Antonio Muiño Miguez, Cecilia Muñoz Delgado, Lucía Ordieres Ortega, Susana Pardo Sánchez, Alejandro Parra Virto, María Teresa Pérez Sanz, Blanca Pinilla Llorente, Sandra Piqueras Ruiz, Guillermo Soria Fernández-Llamazares, María Toledano Macías, Neera Toledo Samaniego, Ana Torres do Rego, Maria Victoria Villalba Garcia, Gracia Villarreal, María Zurita Etayo, Jorge Álvarez Troncoso, Francisco Arnalich Fernández, Francisco Blanco Quintana, Carmen Busca Arenzana, Sergio Carrasco Molina, Aranzazu Castellano Candalija, Germán Daroca Bengoa, Alejandro de Gea Grela, Alicia de Lorenzo Hernández, Alejandro Díez Vidal, Carmen Fernández Capitán, Maria Francisca García Iglesias, Borja González Muñoz, Carmen Rosario Herrero Gil, Juan María Herrero Martínez, Víctor Hontañón, Maria Jesús Jaras Hernández, Carlos Lahoz, Cristina Marcelo Calvo, Juan Carlos Martín Gutiérrez, Monica Martinez Prieto, Elena Martínez Robles, Araceli Menéndez Saldaña, Alberto Moreno Fernández, Jose Maria Mostaza Prieto, Ana Noblejas Mozo, Carlos Manuel Oñoro López, Esmeralda Palmier Peláez, Marina Palomar Pampyn, Maria Angustias Quesada Simón, Juan Carlos Ramos Ramos, Luis Ramos Ruperto, Aquilino Sánchez Purificación, Teresa Sancho Bueso, Raquel Sorriguieta Torre, Clara Itziar Soto Abanedes, Yeray Untoria Tabares, Marta Varas Mayoral, Julia Vásquez Manau, Jose Luis Beato Pérez, Maria Lourdes Sáez Méndez, María Álvarez Bello, Ane Andrés Eisenhofer, Ana Arias Milla, Isolina Baños Pérez, Laura Benítez Gutiérrez, Javier Bilbao Garay, Silvia Blanco Alonso, Jorge Calderón Parra, Alejandro Callejas Díaz, José María Camino Salvador, Mª Cruz Carreño Hernández, Valentín Cuervas-Mons Martínez, Sara de la Fuente Moral, Miguel del Pino Jimenez, Alberto Díaz de Santiago, Itziar Diego Yagüe, Ignacio Donate Velasco, Ana María Duca, Pedro Durán del Campo, Gabriela Escudero López, Esther Expósito Palomo, Ana Fernández Cruz, Esther Fiz Benito, Andrea Fraile López, Amy Galán Gómez, Sonia García Prieto, Claudia García Rodríguez-Maimón, Miguel Ángel García Viejo, Javier Gómez Irusta, Edith Vanessa Gutiérrez Abreu, Isabel Gutiérrez Martín, Ángela Gutiérrez Rojas, Andrea Gutiérrez Villanueva, Jesús Herráiz Jiménez, Pedro Laguna del Estal, Mª Carmen Máinez Sáiz, Cristina Martín Martín, María Martínez Urbistondo, Fernando Martínez Vera, Susana Mellor Pita, Patricia Mills Sánchez, Esther Montero Hernández, Alberto Mora Vargas, Cristina Moreno López, Alfonso Ángel-Moreno Maroto, Victor Moreno-Torres Concha, Ignacio Morrás De La Torre, Elena Múñez Rubio, Ana Muñoz Gómez, Rosa Muñoz de Benito, Alejandro Muñoz Serrano, Jose María Palau Fayós, Lina Marcela Parra Ramírez, Ilduara Pintos Pascual, Arturo José Ramos Martín-Vegue, Antonio Ramos Martínez, Isabel Redondo Cánovas del Castillo, Alberto Roldán Montaud, Lucía Romero Imaz, Yolanda Romero Pizarro, Mónica Sánchez Santiuste, David Sánchez Órtiz, Enrique Sánchez Chica, Patricia Serrano de la Fuente, Pablo Tutor de Ureta, Ángela Valencia Alijo, Mercedes Valentín-Pastrana Aguilar, Juan Antonio Vargas Núñez, Jose Manuel Vázquez Comendador, Gema Vázquez Contreras, Carmen Vizoso Gálvez, Gonzalo Acebes Repiso, Uxua Asín Samper, María Aranzazu Caudevilla Martínez, José Miguel García Bruñén, Rosa García Fenoll, Jesús Javier González Igual, Laura Letona Giménez, Mónica Llorente Barrio, Luis Sáez Comet, María Aguilera García, Ester Alonso Monge, Jesús Álvarez Rodríguez, Claudia Alvarez Varela, Miquel Berniz Gòdia, Marta Briega Molina, Marta Bustamante Vega, Jose Curbelo, Alicia de las Heras Moreno, Ignacio Descalzo Godoy, Alexia Constanza Espiño Alvarez, Ignacio Fernández Martín-Caro, Alejandra Franquet López-Mosteiro, Gonzalo Galvez Marquez, María J. García Blanco, Yaiza García del Álamo Hernández, Clara García-Rayo Encina, Noemí Gilabert González, Carolina Guillamo Rodríguez, Nicolás Labrador San Martín, Manuel Molina Báez, Carmen Muñoz Delgado, Pedro Parra Caballero, Javier Pérez Serrano, Laura Rabes Rodríguez, Pablo Rodríguez Cortés, Carlos Rodriguez Franco, Emilia Roy-Vallejo, Monica Rueda Vega, Aresio Sancha Lloret, Beatriz Sánchez Moreno, Marta Sanz Alba, Jorge Serrano Ballester, Alba Somovilla, Carmen Suarez Fernández, Macarena Vargas Tirado, Almudena Villa Marti, Inés Armenteros Yeguas, Javier Azaña Gómez, Julia Barrado Cuchillo, Irene Burruezo López, Noemí Cabello Clotet, Alberto E. Calvo Elías, Elpidio Calvo Manuel, Carmen María Cano de Luque, Cynthia Chocron Benbunan, Laura Dans Vilan, Ester Emilia Dubon Peralta, Vicente Estrada Pérez, Santiago Fernandez-Castelao, Marcos Oliver Fragiel Saavedra, José Luis García Klepzig, Maria del Rosario Iguarán Bermúdez, Esther Jaén Ferrer, Rubén Ángel Martín Sánchez, Manuel Méndez Bailón, Maria José Nuñez Orantos, Carolina Olmos Mata, Eva Orviz García, David Oteo Mata, Cristina Outon González, Juncal Perez-Somarriba, Pablo Pérez Mateos, Maria Esther Ramos Muñoz, Xabier Rivas Regaira, Iñigo Sagastagoitia Fornie, Alejandro Salinas Botrán, Miguel Suárez Robles, Maddalena Elena Urbano, Miguel Villar Martínez, Nicolás Alcalá Rivera, Anxela Crestelo Vieitez, Esther del Corral Beamonte, Jesús Díez Manglano, Isabel Fiteni Mera, Maria del Mar Garcia Andreu, Martin Gerico Aseguinolaza, Claudia Josa Laorden, Raul Martinez Murgui, Marta Teresa Matía Sanz, Alicia Alonso Álvarez, Olaya Alonso Juarros, Ariadna Arévalo López, Carmen Casariego Castiñeira, Ana Cerezales Calviño, Marta Contreras Sánchez, Ramón Fernández Varela, Santiago J. Freire Castro, Ana Padín Trigo, Rafael Prieto Jarel, Fátima Raad Varea, Ignacio Ramil Freán, Laura Ramos Alonso, Francisco Javier Sanmartín Pensado, David Vieito Porto, Judit Aranda Lobo, Jose Loureiro Amigo, Isabel Oriol Bermúdez, Melani Pestaña Fernández, Nicolas Rhyman, Nuria Vázquez Piqueras, Juan Alberto Aguilera Ayllón, Arturo Artero, María del Mar Carmona Martín, María José Fabiá Valls, Maria de Mar Fernández Garcés, Ana Belén Gómez Belda, Ian López Cruz, Manuel Madrazo López, Elisabet Mateo Sanchis, Jaume Micó Gandia, Laura Piles Roger, Adela Maria Pina Belmonte, Alba Viana García, Maria del Carmen Beceiro Abad, Maria Aurora Freire Romero, Sonia Molinos Castro, Emilio Manuel Paez Guillan, María Pazo Nuñez, Paula Maria Pesqueira Fontan, Sonia Casallo Blanco, Jeffrey Oskar Magallanes Gamboa, Luis Fernando, Abrego Vaca, Ana Andréu Arnanz, Octavio Arce García, Marta Bajo González, Pablo Borque Sanz, Alberto Cozar Llisto, Sonia de Pedro Baena, Beatriz Del Hoyo Cuenda, María Alejandra Gamboa Osorio, Isabel García Sánchez, Andrés González García, Oscar Alberto López Cisneros, Miguel Martínez Lacalzada, Borja Merino Ortiz, Jimena Rey-García, Elisa Riera González, Cristina Sánchez Díaz, Grisell Starita Fajardo, Cecilia Suárez Carantoña, Adrian Viteri Noel, Svetlana Zhilina Zhilina, Carlos Aldasoro Frias, Luis Arribas Perez, María Esther Fraile Villarejo, Beatriz Garcia Lopez, Victor Madrid Romero, Emilia Martínez Velado, Victoria Palomar Calvo, Sara Pintos Otero, Carlota Tuñón de Almeida, Ana Maria Alguacil Muñoz, Marta Blanco Fernández, Veronica Cano, Ricardo Crespo Moreno, Fernando Cuadra Garcia-Tenorio, Blanca Díaz-Tendero Nájera, Raquel Estévez González, María Paz García Butenegro, Alberto Gato Díez, Verónica Gómez Caverzaschi, Piedad María Gómez Pedraza, Julio González Moraleja, Raúl Hidalgo Carvajal, Patricia Jiménez Aranda, Raquel Labra González, Áxel Legua Caparachini, Pilar Lopez Castañeyra, Agustín Lozano Ancin, Jose Domingo Martin Garcia, Cristina Morata Romero, María Jesús Moya Saiz, Helena Moza Moríñigo, Gemma Muñiz Nicolás, Enriqueta Muñoz Platon, Filomena Oliveri, Elena Ortiz Ortiz, Raúl Perea Rafael, Pilar Redondo Galán, María Antonia Sepulveda Berrocal, Vicente Serrano Romero de Ávila, Pilar Toledano Sierra, Yamilex Urbano Aranda, Jesús Vázquez Clemente, Carmen Yera Bergua, Juan Miguel Antón Santos, Ana Belén Barbero Barrera, Coralia Bueno Muiño, Ruth Calderón Hernaiz, Irene Casado Lopez, José Manuel Casas Rojo, Andrés Cortés Troncoso, Mayte de Guzmán García-Monge, Francesco Deodati, Gonzalo García Casasola Sánchez, Elena Garcia Guijarro, Davide Luordo, María Mateos González, Jose A Melero Bermejo, Lorea Roteta García, Elena Sierra Gonzalo, Javier Villanueva Martínez, Ana María Álvarez Suárez, Carlos Delgado Vergés, Rosa Fernandez-Madera Martínez, Eva Fonseca Aizpuru, Alejandro Gómez Carrasco, Cristina Helguera Amezua, Juan Francisco López Caleya, María del Mar Martínez López, Aleida Martínez Zapico, Carmen Olabuenaga Iscar, María Luisa Taboada Martínez, Lara María Tamargo Chamorro, Mª Mar Ayala Gutiérrez, Rosa Bernal López, José Bueno Fonseca, Verónica Andrea Buonaiuto, Luis Francisco Caballero Martínez, Lidia Cobos Palacios, Clara Costo Muriel, Francis de Windt, Ana Teresa Fernandez-Truchaud Christophel, Paula García Ocaña, Ricardo Gómez Huelgas, Javier Gorospe García, Maria Dolores López Carmona, Pablo López Quirantes, Almudena López Sampalo, Elizabeth Lorenzo Hernández, Juan José Mancebo Sevilla, Jesica Martin Carmona, Luis Miguel Pérez-Belmonte, Araceli Pineda Cantero, Carlos Romero Gómez, Michele Ricci, Jaime Sanz Cánovas, Marisa Asensio Tomás, David Balaz, David Bonet Tur, Ruth Cañizares Navarro, Paloma Chazarra Pérez, Jesús Corbacho Redondo, Leticia Espinosa Del Barrio, Pedro Jesús Esteve Atiénzar, Carles García Cervera, David Francisco García Núñez, Vicente Giner Galvañ, Angie Gómez Uranga, Javier Guzmán Martínez, Isidro Hernández Isasi, Lourdes Lajara Villar, Juan Manuel Núñez Cruz, Sergio Palacios Fernández, Juan Jorge Peris García, Andrea Riaño Pérez, José Miguel Seguí Ripoll, Azucena Sempere Mira, Philip Wikman-Jorgensen, Jesús Ballano Rodríguez-Solís, Luis Cabeza Osorio, María del Pilar Fidalgo Montero, Mª Isabel Fuentes Soriano, Erika Esperanza Lozano Rincon, Ana Martín Hermida, Jesus Martinez Carrilero, Jose Angel Pestaña Santiago, Manuel Sánchez Robledo, Patricia Sanz Rojas, Nahum Jacobo Torres Yebes, Vanessa Vento, Dafne Cabañero, María Calabuig Ballester, Pascual Císcar Fernández, Ricardo Gil Sánchez, Marta Jiménez Escrig, Cristina Marín Amela, Laura Parra Gómez, Carlos Puig Navarro, José Antonio Todolí Parra, Diana Alegre González, Irene Ariño Pérez de Zabalza, Sergio Arnedo Hernández, Jorge Collado Sáenz, Beatriz Dendariena, Marta Gómez del Mazo, Iratxe Martínez de Narvajas Urra, Sara Martínez Hernández, Estela Menendez Fernández, Jose Luís Peña Somovilla, Elisa Rabadán Pejenaute, Raquel Aranega González, Ramon Boixeda, Javier Fernández Fernández, Carlos Lopera Mármol, Marta Parra Navarro, Ainhoa Rex Guzmán, Aleix Serrallonga Fustier, Antonio Pablo Arenas de Larriva, Pilar Calero Espinal, Javier Delgado Lista, Francisco Fuentes-Jiménez, María Jesús Gómez Vázquez, Jose Jiménez Torres, José López-Miranda, Laura Martín Piedra, Javier Pascual Vinagre, Pablo Pérez-Martinez, María Elena Revelles Vílchez, Juan Luis Romero Cabrera, José David Torres Peña, Francisco Javier Bejarano Luque, Francisco Javier Carrasco-Sánchez, Mercedes de Sousa Baena, Jaime Díaz Leal, Aurora Espinar Rubio, Maria Franco Huertas, Juan Antonio García Bravo, Andrés Gonzalez Macías, Encarnación Gutiérrez Jiménez, Alicia Hidalgo Jiménez, Constantino Lozano Quintero, Carmen Mancilla Reguera, Francisco Javier Martínez Marcos, Francisco Muñoz Beamud, Maria Perez Aguilera, Alícia Perez Jiménez, Virginia Rodríguez Castaño, Alvaro Sánchez de Alcazar del Río, Leire Toscano Ruiz, María Esther Guisado Espartero, Lorena Montero Rivas, Maria de la Sierra Navas Alcántara, Raimundo Tirado-Miranda, Begoña Cortés Rodríguez, Victoria Augustín Bandera, María Dolores Martín Escalante, Pablo Conde Baena, Joaquin Escobar Sevilla, Laura Gallo Padilla, Patricia Gómez Ronquillo, Pablo González Bustos, María Navío Botías, Jessica Ramírez Taboada, Mar Rivero Rodríguez, Hortensia Alvarez Diaz, Tamara Dalama Lopez, Estefania Martul Pego, Carmen Mella Pérez, Ana Pazos Ferro, Sabela Sánchez Trigo, Dolores Suarez Sambade, Maria Trigas Ferrin, Maria del Carmen Vázquez Friol, Laura Vilariño Maneiro, Rosario Maria García Diez, Manuel Martin Regidor, Angel Luis Martínez Gonzalez, Alberto Muela Molinero, Raquel Rodríguez Díez, Beatriz Vicente Montes, Raquel Fernández González, Amara Gonzalez Noya, Carlos Hernández Ceron, Isabel Izuzquiza Avanzini, Ana Latorre Diez, Pablo López Mato, Ana María Lorenzo Vizcaya, Daniel Peña Benítez, Milagros María Peña Zemsch, Lucía Pérez Expósito, Marta Pose Bar, Lara Rey González, Laura Rodrigo Lara, Javier Ena, Jose Enrique Gómez Segado, Luis Felipe Díez García, Iris El Attar Acedo, Bárbara Hernandez Sierra, Carmen Mar Sánchez Cano, Yolanda Casillas Viera, Lucía Cayuela Rodríguez, Carmen de Juan Alvarez, Gema Flox Benitez, Laura García Escudero, Juan Martin Torres, Patricia Moreira Escriche, Susana Plaza Canteli, M. Carmen Romero Pérez, Ana Suarez Lombraña, Sara Fuente Cosío, César Manuel Gallo Álvaro, Julia Lobo García, Antía Pérez Piñeiro, Rafael Aragon Lara, Inmaculada Cimadevilla Fernandez, Juan Carlos Cira García, Gema Maria García García, Julia Gonzalez Granados, Beatriz Guerrero Sánchez, Francisco Javier Monreal Periáñez, Maria Josefa Pascual Perez, Alba Camarena Molina, Simona Cioaia, Anna Ferrer Santolalia, José María Frutos Pérez, Eva Gil Tomás, Leyre Jorquer Vidal, Marina Llopis Sanchis, M Ángeles Martínez Pascual, Alvaro Navarro Batet, Mari Amparo Perea Ribis, Ricardo Peris Sanchez, José Manuel Querol Ribelles, Silvia Rodriguez Mercadal, Ana Ventura Esteve, Jorge Andrés Soler, Marián Bennasar Remolar, Alejandro Cardenal Álvarez, Daniela Díaz Carlotti, María José Esteve Gimeno, Sergio Fabra Juana, Paula García López, María Teresa Guinot Soler, Daniela Palomo de la Sota, Guillem Pascual Castellanos, Ignacio Pérez Catalán, Celia Roig Martí, Paula Rubert Monzó, Javier Ruiz Padilla, Nuria Tornador Gaya, Jorge Usó Blasco, Gloria María Alonso Claudio, Víctor Barreales Rodríguez, Cristina Carbonell Muñoz, Adela Carpio Pérez, María Victoria Coral Orbes, Daniel Encinas Sánchez, Sandra Inés Revuelta, Miguel Marcos Martín, José Ignacio Martín González, José Ángel Martín Oterino, Leticia Moralejo Alonso, Sonia Peña Balbuena, María Luisa Pérez García, Ana Ramon Prados, Beatriz Rodríguez-Alonso, Ángela Romero Alegría, Maria Sanchez Ledesma, Rosa Juana Tejera Pérez, Anyuli Gracia Gutiérrez, Leticia Esther Royo Trallero, Pablo Guisado Vasco, Ana Roda Santacruz, Ana Valverde Muñoz, Francisco Epelde, Isabel Torrente, Maricruz Almendros Rivas, Miquel Hortos Alsina, Anabel Martin-Urda Diez-Canseco, Mª José Esteban Giner, Xjoylin Teresita Egües Torres, Sara Gutiérrez González, Cristina Novoa Fernández, Pablo Tellería Gómez, Virginia Herrero García, Berta Román Bernal, Vanesa Alende Castro, Ana María Baz Lomba, Ruth Brea Aparicio, Marta Fernandez Morales, Jesus Manuel Fernandez Villar, Maria Teresa Lopez Monteagudo, Cristina Pérez García, Lorena María Rodríguez Ferreira, Diana Sande Llovo, Maria Begoña Valle Feijoo, José López Castro, Manuel Lorenzo López Reboiro
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Funding
This research received no external funding.
Author contributions
Conceptualization, JDTP, LMPB, JMRR, and RGH; data curation, JDTP, LMPB, FFJ, MDLC, PPM, JLM, FJCS, JAVN, ECB,JOMG, AGG, JGM, ACT, MLTM, MPFM, JMSR, RGS, DAG, RB, BCR, JE, GMGG, AVE; formal analysis, JDTP, LMPB; investigation, JDTP, LMPB; methodology, JDTP, LMPB, JMRR, and RGH; project administration, JMCR and RGH; resources, JDTP and LMPB; software, JDTP; supervision, JMRR and RGH; validation,, JDTP and LMPB; visualization, JDTP and LMPB; writing—original draft, JDTP and LMPB and FFJ; writing—review and editing, JDTP, LMPB, FFJ, PPM, JMRR, and RGH; and funding acquisition, none All authors have read and agreed to the published version of the manuscript.
Additional information
The members of the SEMI-COVID-19 Network are mentioned in Acknowledgements section.
Rights and permissions
About this article
Cite this article
Torres-Peña, J.D., Pérez-Belmonte, L.M., Fuentes-Jiménez, F. et al. Prior Treatment with Statins is Associated with Improved Outcomes of Patients with COVID-19: Data from the SEMI-COVID-19 Registry. Drugs 81, 685–695 (2021). https://doi.org/10.1007/s40265-021-01498-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01498-x