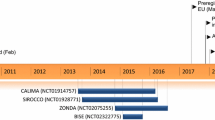
Abstract
Thymic stromal lymphopoietin (TSLP) is an epithelial cell-derived cytokine implicated in the pathogenesis of asthma. Tezepelumab (tezepelumab-ekko; TEZSPIRE™) is a first-in-class human IgG2λ monoclonal antibody that inhibits the action of TSLP. Administered subcutaneously, it is being developed by Amgen and AstraZeneca for the treatment of asthma, chronic obstructive pulmonary disease (COPD), chronic rhinosinusitis with nasal polyps (CRSwNP), chronic spontaneous urticaria and eosinophilic oesophagitis. Tezepelumab received its first approval on 17 December 2021 as an add-on maintenance treatment for patients aged ≥ 12 years with severe asthma in the USA; it is the only biologic approved for severe asthma with no phenotype (e.g. eosinophilic or allergic) or biomarker limitations. A regulatory assessment of tezepelumab for the treatment of asthma is currently underway in the EU and Japan. Tezepelumab received orphan drug designation for the treatment of eosinophilic oesophagitis in October 2021 in the USA, and is undergoing clinical development for the treatment of COPD, CRSwNP and chronic spontaneous urticaria. This article summarizes the milestones in the development of tezepelumab leading to this first approval for the add-on maintenance treatment of patients aged ≥ 12 years with severe asthma.
Similar content being viewed by others
References
McGregor MC, Krings JG, Nair P, et al. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433–45.
Global Initiative for Asthma. 2021 GINA Report, Global Strategy for Asthma Management and Prevention. 2021. https://ginasthma.org/gina-reports/. Accessed 1 Feb 2022.
Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–33.
AstraZeneca AB, Amgen Inc. TEZSPIRE™ (tezepelumab-ekko) injection, for subcutaneous use: US prescribing information. 2021. https://www.fda.gov/. Accessed 20 Dec 2021.
Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–9.
AstraZenaca. Tezspire (tezepelumab) approved in the US for severe asthma [media release]. 17 Dec 2021. https://www.astrazeneca.com/.
Amgen. Amgen reports second quarter 2021 financial results [media release]. 3 Aug 2021. http://www.amgen.com.
AstraZeneca. Tezepelumab granted Orphan Drug Designation in the US for eosinophilic esophagitis [media release]. 8 Oct 2021. http://www.astrazeneca-us.com.
Amgen, AstraZeneca. Amgen and AstraZeneca announce collaboration to jointly develop and commercialize clinical-stage inflammation portfolio [media release]. 2 Apr 2012. http://www.astrazeneca.com.
AstraZeneca. Tezepelumab NAVIGATOR phase III trial met primary endpoint of a statistically significant and clinically meaningful reduction in exacerbations in a broad population of patients with severe asthma [media release]. 10 Nov 2020. http://www.astrazeneca.com.
Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299–312.
Corren J, Pham TH, Gil EG, et al. Baseline type 2 biomarker levels and response to tezepelumab in severe asthma. Allergy. 2021. https://doi.org/10.1111/all.15197.
Wechsler M, Menzies Gow A, Brightling CE, et al. Reductions in inflammatory biomarkers in patients with oral corticosteroid-dependent asthma treated with tezepelumab [abstract no. A1454]. Am J Respir Crit Care Med. 2021;203(9).
Pavord ID, Menzies Gow A, Bengtsson T, et al. Eosinophil counts and fractional exhaled nitric oxide levels after cessation of tezepelumab: results from the PATHWAY phase 2b study [abstract no. A4255]. Am J Respir Crit Care Med. 2020;201(1).
Pham TH, Chen C, Colice G, et al. Tezepelumab normalizes serum interleukin-5 and -13 levels in patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol. 2021;127(6):689–91.
Sridhar S, Zhao W, Pham TH, et al. Tezepelumab decreases matrix remodelling and inflammatory pathways in patients with asthma [abstract no. RCT3785]. Eur Respir J. 2019;54(Suppl 63).
Ly N, Zheng Y, Griffiths JM, et al. Pharmacokinetic and pharmacodynamic modeling of tezepelumab to guide phase 3 dose selection for patients with severe asthma. J Clin Pharmacol. 2021;61(7):901–12.
Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370(22):2102–10.
Sverrild A, Hansen S, Hvidtfeldt M, et al. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur Respir J. 2021;59(1):2101296.
Sverrild A, Cerps S, Nieto-Fontarigo J, et al. Effects of tezepelumab on host epithelial tolerance to virus in patients with uncontrolled asthma [abstract no. OA1492]. Eur Respir J. 2021;58(Suppl 65).
Alpizar S, Megally A, Chen C, et al. Functionality and performance of an accessorized pre-filled syringe and an autoinjector for at-home administration of tezepelumab in patients with severe, uncontrolled asthma. J Asthma Allergy. 2021;14:381–92.
Sakamoto K, Matsuki S, Irie S, et al. A phase 1, randomized, placebo-controlled study to evaluate the safety, tolerability, pharmacokinetics, and immunogenicity of subcutaneous tezepelumab in healthy Japanese men. Clin Pharmacol Drug Dev. 2020;9(7):833–40.
Zheng Y, Abuqayyas L, Megally A, et al. Tezepelumab pharmacokinetics, safety, and tolerability after administration via vial-and-syringe, accessorized prefilled syringe, or autoinjector: a randomized trial in healthy volunteers. Clin Ther. 2021;43(1):142-55.e5.
Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–46.
Corren J, Chen S, Callan L, et al. The effect of tezepelumab on hospitalizations and emergency department visits in patients with severe asthma. Ann Allergy Asthma Immunol. 2020;125(2):211–4.
Wechsler ME, Colice G, Griffiths JM, et al. SOURCE: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir Res. 2020;21(1):264.
Wechsler M, Gow AM, Brightling CE, et al. Oral corticosteroid-sparing effect of tezepelumab in adults with severe asthma [abstract no. A1197]. Am J Respir Crit Care Med. 2021;203.
Corren J, Wheatley L, Sanda S, et al. Effects of combined treatment with cat allergen immunotherapy and tezepelumab on nasal allergen challenge [abstract no. AB336]. J Allergy Clin Immunol. 2020;145(2 Suppl).
US National Institutes of Health. ClinicalTrials.gov identifier NCT02237196. 2020. https://clinicaltrials.gov/. Accessed 21 Jan 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. S. M. Hoy is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hoy, S.M. Tezepelumab: First Approval. Drugs 82, 461–468 (2022). https://doi.org/10.1007/s40265-022-01679-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01679-2