Abstract
Background
Metastatic cancers occur when cancer cells break away from the primary tumour. One of the most common sites of metastasis is the bone, with several therapeutic options currently available for managing bone metastases. In a resource-constrained environment, policy makers and practitioners need to know which options are cost effective.
Objective
The aim of this systematic review was to review and appraise published economic evaluations on treatments for the management of bone metastases.
Methods
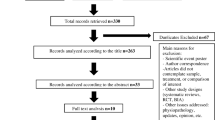
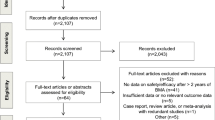
We searched eight bibliographic databases (MEDLINE, MEDLINE in Process, EMBASE, CSDR, DARE, HTA, EED and CPCI) for relevant economic evaluations published from each database’s inception date until March 2017. Study selection, quality assessment and data extraction were carried out according to published guidelines.
Results
Twenty-four relevant economic analyses were identified. Seventeen of these studies focused on bone metastases resulting from a particular type of cancer, i.e. prostate (n = 8), breast (n = 7), lung (n = 1) or renal (n = 1), while seven report results for various primary tumours. Across types of cancer, evidence suggests that bisphosphonates result in lower morbidity and improved quality of life, for an additional cost, which is typically below conventional cost-effectiveness thresholds. While denosumab leads to health gains compared with zoledronic acid, it also results in substantial additional costs and is unlikely to represent value for money. The limited literature on the radiopharmaceutical strontium-89 (Sr89) and external beam radiotherapy (EBR) suggest that these treatments are cost effective compared with no treatment.
Conclusions
The reviewed evidence suggests that bisphosphonate treatments are cost-effective options for bone metastases, while denosumab is unlikely to represent value for money. Evidence on EBR and Sr89 is limited and less conclusive.




Similar content being viewed by others
References
Cancer Research UK. Worldwide Cancer Statistics 2017. http://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer#heading-Zero. Accessed 10 Aug 2017.
Cancer Research UK. Cancer incidence statistics 2017. Available at: http://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence#heading-Zero. Accessed 10 Aug 2017.
Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602.
Cancer Research UK. About secondary bone cancer 2017. Available at: http://www.cancerresearchuk.org/about-cancer/secondary-cancer/secondary-bone-cancer/about. Accessed 10 Aug 2017.
Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20):6243s–9s.
Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;8:584–93.
Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9(3):509–24.
Nilsson S, Larsen RH, Fossa SD, Balteskard L, Borch KW, Westlin JE, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res. 2005;11(12):4451–9.
Pandit-Taskar N, Batraki M, Divgi CR. Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J Nucl Med. 2004;45(8):1358–65.
Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032–45.
Body JJ, Lipton A, Gralow J, Steger GG, Gao G, Yeh H, et al. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J Bone Miner Res. 2010;25(3):440–6.
Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–22.
NHS Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: NHS Centre for Reviews and Dissemination, University of York; 2009.
Higgins JPTG, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0: The Cochrane Collaboration; 2011. Available at: http://handbook-5-1.cochrane.org/. Accessed 8 Jan 2017.
Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8):1588–94.
NHS CRD. Search strategies. York: NHS Centre for Reviews and Dissemination; 2015. Available at: http://www.crd.york.ac.uk/crdweb/searchstrategies.asp. Accessed 10 Jan 2017.
Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care. 2005;21(2):240–5.
Andronis L, Goranitis I, Pirrie S, Pope A, Barton D, Collins S, et al. Cost-effectiveness of zoledronic acid and strontium-89 as bone protecting treatments in addition to chemotherapy in patients with metastatic castrate-refractory prostate cancer: results from the TRAPEZE trial (ISRCTN 12808747). Br J Urol Int. 2017;119(4):522–9.
Botteman M, Barghout V, Stephens J, Hay J, Brandman J, Aapro M. Cost effectiveness of bisphosphonates in the management of breast cancer patients with bone metastases. Ann Oncol. 2006;17(7):1072–82.
Botteman MF, Meijboom M, Foley I, Stephens JM, Chen YM, Kaura S. Cost-effectiveness of zoledronic acid in the prevention of skeletal-related events in patients with bone metastases secondary to advanced renal cell carcinoma: application to France, Germany, and the United Kingdom. Eur J Health Econ. 2011;12(6):575–88.
Carter JA, Joshi A, Kaura S, Botteman MF. Cost effectiveness of zoledronic acid in the management of skeletal metastases in hormone-refractory prostate cancer patients in France, Germany, Portugal, and the Netherlands. J Med Econ. 2011;14(3):288–98.
Collinson L, Kvizhinadze G, Nair N, McLeod M, Blakely T. Economic evaluation of single-fraction versus multiple-fraction palliative radiotherapy for painful bone metastases in breast, lung and prostate cancer. J Med Imaging Radiat Oncol. 2016;60(5):650–60.
De Cock E, Hutton J, Canney P, Body JJ, Barrett-Lee P, Neary MP, et al. Cost-effectiveness of oral ibandronate versus IV zoledronic acid or IV pamidronate for bone metastases in patients receiving oral hormonal therapy for breast cancer in the United Kingdom. Clin Ther. 2005;27(8):1295–310.
De Cock E, Hutton J, Canney P, Body JJ, Barrett-Lee P, Neary MP, et al. Cost-effectiveness of oral ibandronate compared with intravenous (i.v.) zoledronic acid or i.v. generic pamidronate in breast cancer patients with metastatic bone disease undergoing i.v. chemotherapy. Support Care Cancer. 2005;13(12):975–86.
Dranitsaris G, Hsu T. Cost utility analysis of prophylactic pamidronate for the prevention of skeletal related events in patients with advanced breast cancer. Support Care Cancer. 1999;7(4):271–9.
Ford J, Cummins E, Sharma P, Elders A, Stewart F, Johnston R, et al. Systematic review of the clinical effectiveness and cost-effectiveness, and economic evaluation, of denosumab for the treatment of bone metastases from solid tumours. Health Technol Assess. 2013;17(29):1–386.
Gessner U, Koeberle D, Thuerlimann B, Bacchus L, Horisberger B. Economic analysis of terminal care for patients with malignant osteolytic bone disease and pain treated with pamidronate. Support Care Cancer. 2000;8(2):115–22.
Hillner BE, Weeks JC, Desch CE, Smith TJ. Pamidronate in prevention of bone complications in metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol. 2000;18(1):72–9.
James N, Pirrie S, Pope A, Barton D, Andronis L, Goranitis I, et al. TRAPEZE: a randomised controlled trial of the clinical effectiveness and cost-effectiveness of chemotherapy with zoledronic acid, strontium-89, or both, in men with bony metastatic castration-refractory prostate cancer. Health Technol Assess. 2016;20(53):1–127.
Joshi AD, Carter JA, Botteman MF, Kaura S. Cost-effectiveness of zoledronic acid in the management of skeletal metastases in patients with lung cancer in France, Germany, Portugal, the Netherlands, and the United Kingdom. Clin Ther. 2011;33(3):291–304.
Konski A. Radiotherapy is a cost-effective palliative treatment for patients with bone metastasis from prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60(5):1373–8.
McEwan AJ, Amyotte GA, McGowan DG, MacGillivray JA, Porter AT. A retrospective analysis of the cost effectiveness of treatment with Metastron (89Sr-chloride) in patients with prostate cancer metastatic to bone. Nuclear Med Commun. 1994;15(7):499–504.
Reed SD, Radeva JI, Glendenning GA, Saad F, Schulman KA. Cost-effectiveness of zoledronic acid for the prevention of skeletal complications in patients with prostate cancer [structured abstract]. J Urol. 2004;171(4):1537–42.
Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al. A systematic review of the role of bisphosphonates in metastatic disease. Health Technol Assess. 2004;8(4):1–176.
Snedecor SJ, Carter JA, Kaura S, Botteman MF. Cost-effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin Ther. 2012;34(6):1334–49.
Snedecor SJ, Carter JA, Kaura S, Botteman MF. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a cost-effectiveness analysis. J Med Econ. 2013;16(1):19–29.
Stopeck A, Rader M, Henry D, Danese M, Halperin M, Cong Z, et al. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ. 2012;15(4):712–23.
van den Hout WB, van der Linden YM, Steenland E, Wiggenraad RG, Kievit J, de Haes H, et al. Single-versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95(3):222–9.
Xie J, Diener M, Sorg R, Wu EQ, Namjoshi M. Cost-effectiveness of denosumab compared with zoledronic acid in patients with breast cancer and bone metastases. Clin Breast Cancer. 2012;12(4):247–58.
Xie J, Namjoshi M, Wu EQ, Parikh K, Diener M, Yu AP, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J Managed Care Pharma. 2011;17(8):621–43.
Yfantopoulos J, Christopoulou A, Chatzikou M, Fishman P, Chatzaras A. The importance of economic evaluation in healthcare decision-making: a case of denosumab versus zoledronic acid from Greece. Third-party payer perspective. Forum Clin Oncol. 2013;4(2):25–31.
Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices—overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 2012;15(6):796–803.
NICE. NICE Guide to the methods of technology appraisal. National Institute for Health and Care Excellence; 2013. Available at: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed 16 Jan 2017.
Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
James ND, Pirrie SJ, Pope AM, Barton D, Andronis L, Goranitis I, et al. Clinical outcomes and survival following treatment of metastatic castrate-refractory prostate cancer with docetaxel alone or with strontium-89, zoledronic acid, or both: the TRAPEZE randomized clinical trial. JAMA Oncol. 2016;2(4):493–9.
Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–9.
Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer. 2004;100(12):2613–21.
Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clinic Oncol. 2003;21(16):3150–7.
Nimdet K, Chaiyakunapruk N, Vichansavakul K, Ngorsuraches S. A systematic review of studies eliciting willingness-to-pay per quality-adjusted life year: does it justify CE threshold? PLoS One. 2015;10(4):e0122760.
Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7.
Brazier J, Ratcliffe J, Saloman J, Tsuchiya A. Measuring and valuing health benefits for economic evaluation. Oxford: Oxford University Press; 2007.
Garau M, Shah KK, Mason AR, Wang Q, Towse A, Drummond MF. Using QALYs in cancer. Pharmacoeconomics. 2011;29(8):673–85.
Devlin NJ, Lorgelly PK. QALYs as a measure of value in cancer. J Cancer Policy. 2017;11:19–25.
Griffin SC, Claxton KP, Palmer SJ, Sculpher MJ. Dangerous omissions: the consequences of ignoring decision uncertainty. Health Econ. 2011;20(2):212–24.
Andronis L. Analytic approaches for research priority-setting: issues, challenges and the way forward. Expert Rev Pharmacoecon Outcomes Res. 2015;15(5):745–54.
Fleurence RL, Torgerson DJ. Setting priorities for research. Health Policy. 2004;69(1):1–10.
Wilson EC. A practical guide to value of information analysis. Pharmacoeconomics. 2015;33(2):105–21.
Steuten L, van de Wetering G, Groothuis-Oudshoorn K, Retel V. A systematic and critical review of the evolving methods and applications of value of information in academia and practice. Pharmacoeconomics. 2013;31(1):25–48.
Thorn J, Coast J, Andronis L. Interpretation of the expected value of perfect information and research recommendations: a systematic review and empirical investigation. Med Decis Mak. 2016;36(3):285–95.
Tuffaha HW, Gordon LG, Scuffham PA. Value of information analysis in oncology: the value of evidence and evidence of value. J Oncol Pract. 2014;10(2):e55–62.
Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S. A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme. Health Technol Assess. 2004;8(31):1–103, iii.
Strong M, Oakley JE, Brennan A, Breeze P. Estimating the expected value of sample information using the probabilistic sensitivity analysis sample: a fast, nonparametric regression-based method. Med Decis Mak. 2015;35(5):570–83.
Andronis L, Billingham LJ, Bryan S, James ND, Barton PM. A practical application of value of information and prospective payback of research to prioritize evaluative research. Med Decis Mak. 2016;36(3):321–34.
Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol. 2002;31(1):115–23.
Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–44.
Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–43.
Author information
Authors and Affiliations
Contributions
LA, IG and RD contributed to the study conception and design, article selection, data extraction, data interpretation and manuscript preparation. SB contributed to the study conception, designed and conducted search strategies, retrieved identified articles and contributed to manuscript preparation. All authors approved the final version of this article.
Corresponding author
Ethics declarations
Conflict of interest
Lazaros Andronis, Ilias Goranitis, Sue Bayliss and Rui Duarte declare no conflicts of interest.
Funding
No funding has been received for this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Andronis, L., Goranitis, I., Bayliss, S. et al. Cost-Effectiveness of Treatments for the Management of Bone Metastases: A Systematic Literature Review. PharmacoEconomics 36, 301–322 (2018). https://doi.org/10.1007/s40273-017-0595-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-017-0595-0